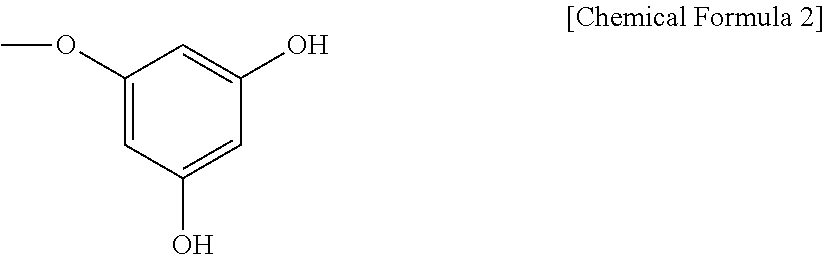Compositions for reducing beta-amyloid-induced neurotoxicity comprising beta-secretase inhibitor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
Preparation of Primary 1,3,5-Trihydroxybenzene Polymer Product
[0043]300 g of 1,3,5-trihydroxybenzene was subject to polymerization at 230° C. for 1 hr under the condition of a water content of 5, 10, 20 or 50 wt % and a pressure of 0.1, 1, 3, 10 or 100 mmHg. Each product was extracted with 1 L of 80% ethanol to remove insoluble, matter therefrom, followed by drying in a vacuum to give a black solid. The solid was washed with distilled water and recovered in a n-butanol layer to yield primary 1,3,5-trihydroxybenzene polymer products.
experimental example 1
Assay of Primary 1,3,5-Trihydroxybenzene Polymer Product for Inhibitory Activity Against B-Secretase
[0044]The primary 1,3,5-trihydroxybenzene polymer products obtained under the various conditions were assayed for inhibitory activity against β-secretase. For this purpose, a human recombinant BACE1 assay kit (PanVera, Wis., USA) was used according to the manufacturer's instructions.
[0045]10 μL of a substrate (75 μM Rh-EVNLDAEFK-Quencher in 50 mM ammonium bicarbonate) was mixed with 10 μL of the enzyme (BACE1, 1 U / mL), 10 μL of an assay buffer, 10 μL of a sample solution (a solution of the primary 1,3,5-trihydroxybenzene polymer product in an assay buffer) to give a reaction mixture in which the sample was contained in a concentration of 1 mg / mL. For a negative control, an assay buffer containing none of the samples was used instead of the sample solution. In the absence of light, each reaction mixture was allowed to react at 25° C. for 60 min. Thereafter, while a light beam at 528 nm...
preparation example 2
Separation of Dibenzofuran Derivative from Mix Sample #1
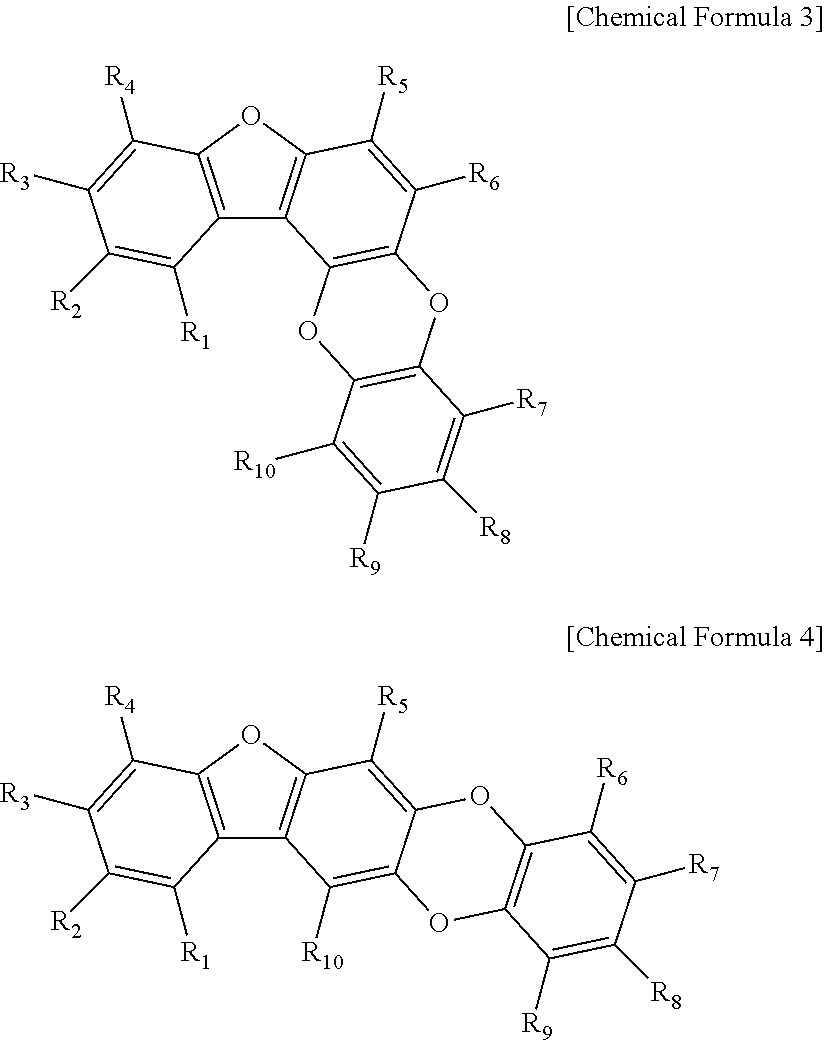
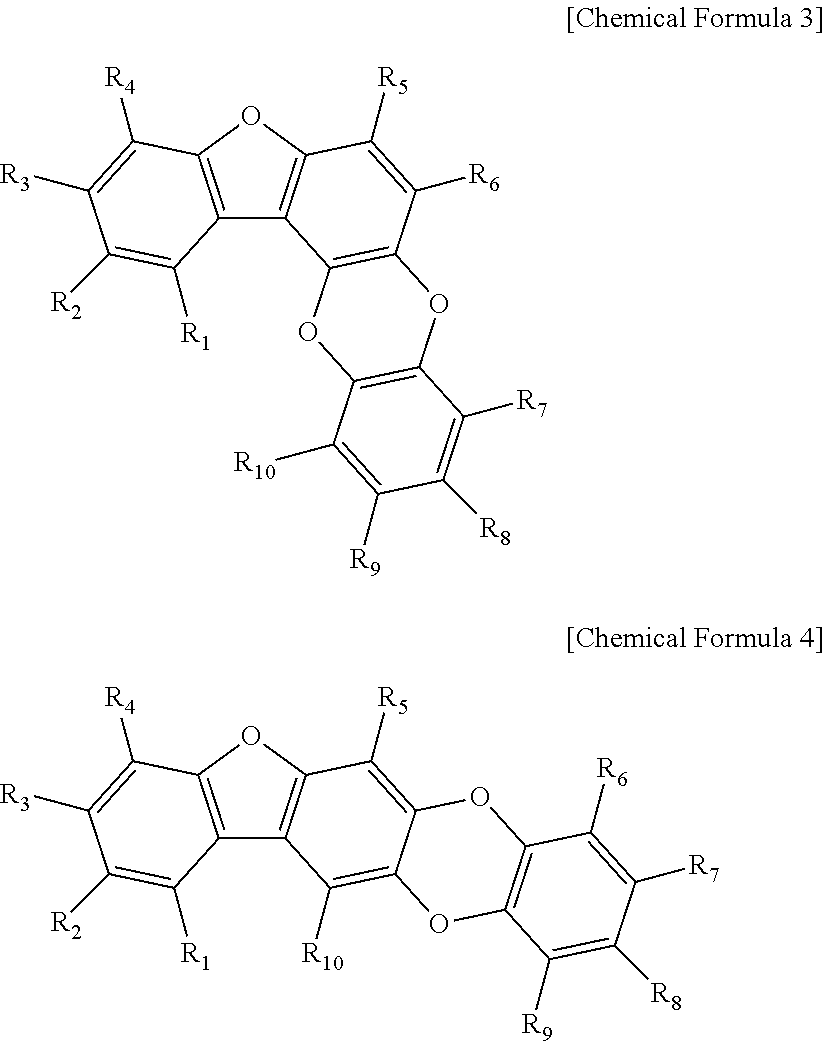
[0052]50 g of the mix sample #1 was loaded onto a liquid chromatography column. Liquid chromatography was performed by eluting with a linear gradient of from 15% to 70% methanol solution over 30 min at a flow rate of 1.0 mL / min on an HP ODS Hypersil column to obtain 11 main fractions (fraction #1-1 to #1-11). The 11 main fractions, each adjusted to a final concentration of 10 μg / mL, were screened for 50% or higher BACE inhibition. Fraction #1-8 (3.5 g) was measured to show the highest inhibitory activity. In order to identify the components of fraction #1-8, 100 mg of fraction #1-8 was subjected to HPLC [Waters Spherisorb S10 ODS2 column (20×250 mm), eluent: 30% MeOH, flow rate: 3.5 ml / min] to separate four active substances. Their structures were determined by 500 MHz 1H- and 125 MHz 13C-NMR to spectrometry (JEOL ECP-500 FT-NMR, JEOL, Japan) and FABMS (VG Autospec Ultima mass spectrometer), with TMS used as the internal standa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com