Traditional Chinese medicine composition for treating rheumatoid arthritis and preparation method thereof
A rheumatoid and arthritis technology, applied in the field of traditional Chinese medicine composition and its preparation for the treatment of rheumatoid arthritis, can solve the problems of endangering the health and quality of life of patients, heavy burdens on society and families, and achieve good social value and economic benefits , low cost of treatment, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
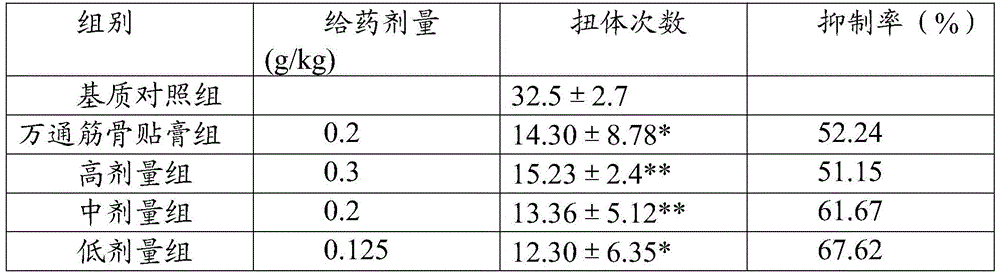
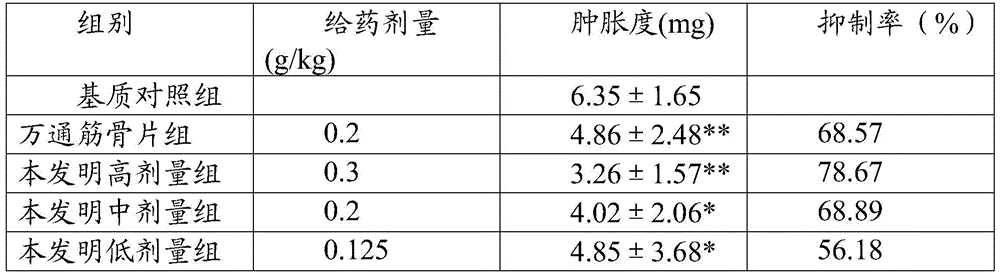
Examples
preparation example Construction
[0078] The preparation method of honey refining pill of the present invention:
[0079] 1500g Chuanduan, 1500g rehmannia, 1500g papaya, 1500g angelica, 1500g drynaria, 1500g poria, 1500g earthworm, 1500g psoralen, 1500g frankincense, 1500g chuanxiong, 1500g atractylodes, 1500g mulberry, 1500g corydalis, Zhishou 1500g of blackberry, 1500g of mulberry, 1500g of dogwood, 1500g of Caulis Spatholobus, 1500g of cloves, and 1500g of Achyranthes bidentata, put the crude drug into pulverizer and cross 120 mesh fine sieves after pulverizing, so that the amount of fine powder that can pass through 120 mesh sieves reaches 20%, collect the crude drug fine powder passing through 120 mesh sieves for subsequent use. Put the remaining coarse particles after sieving in the above steps into ethanol, soak for more than 2 hours, add 8 times the amount of ethanol and 6 times the total mass of the raw material drug, and heat and extract twice, and the extraction time is 2 hours, 1 hour. hour; combi...
experiment example 2
[0094] Experimental example 2: Cumulative toxicity test:
[0095] Traditional Chinese medicine preparation of the present invention is taken orally honey refined pills to mice by 7.69, 19.18 and 43.21g crude drug / kg continuous medication 15 weeks (1.0ml / 100g body weight, every day 2 times) and after drug withdrawal 3 weeks, the result shows: Chinese medicine of the present invention The preparation has no obvious effect on the hair, behavior, defecation, body weight, organ weight, blood picture, liver and kidney function, blood sugar, blood lipid and other indicators of rats. After 1 week and 3 weeks after stopping the drug, there was no significant change in the organs of the rats. It shows that the oral capsule of the traditional Chinese medicine preparation of the present invention has little toxicity to rats after long-term administration, and there is no abnormal reaction after drug withdrawal, so the application is safe.
experiment example 3
[0096] Experimental example 3: skin irritation test
[0097] Rabbits were taken to shave, and the external drug cream was subjected to induction and stimulation contact tests. After 24 hours and 48 hours, the test rabbits were observed, and there was no phenomenon such as erythema and edema in the skin, and there was no difference with the negative control group. The results show that the preparation has close to zero skin reaction intensity, that is, no skin allergy. The irritation test was carried out on the vagina and damaged skin of white mice, and no irritation reaction and other adverse effects were found. Toxicity tests have proved that the drug has no toxic effects and adverse reactions, and is safe and reliable in clinical application.
[0098] Acute percutaneous toxicity test, skin irritation test, skin allergy test, anti-inflammatory test, analgesic test, microcirculation test have been carried out to this medicinal preparation for external use, and its research re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com