Method for preparing lorcaserin
A technology of green caserin and chlorophenyl, which is applied in the field of medicine, can solve problems such as potential safety hazards, cumbersome process operations, shortening the reaction time of Fuckers alkylation, etc., and achieve the effect of ensuring quality and safe and simple post-treatment process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
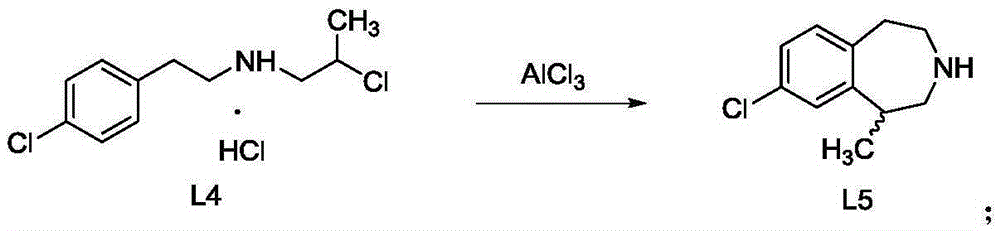
[0023] The invention provides a preparation method of green caserin, comprising:
[0024] A) Using 1-[[2-(4-chlorophenyl)ethyl]amino]-2-chloropropane hydrochloride as a raw material, a green caserin racemate was synthesized by a solvent-free method;
[0025] B) Cool the reaction system to 70-80°C, add an organic solvent, stir evenly and cool to 0-5°C; the organic solvent is tetrachloroethylene, chlorobenzene, toluene or cyclohexane;
[0026] C) adding hydrochloric acid solution to the reaction system, separating liquids, removing the organic phase, and obtaining the aqueous phase;
[0027] D) adding NaOH solution to the above water phase, then adding cyclohexane or n-heptane for extraction, and concentrating the organic phase to obtain pure green caserin racemate.
[0028] After the solvent-free alkylation reaction, the present invention adds organic solvents such as tetrachlorethylene, chlorobenzene, toluene or cyclohexane, which solves the safety problem caused by the rapid...
Embodiment 1
[0055] 1-[[2-(4-chlorophenyl)ethyl]amino]-2-chloropropane hydrochloride 100g, anhydrous AlCl 3 (1.5eq.) 75.2g was added to (500mL) four-neck flask in turn, started stirring and heating, and raised the temperature to about 120°C for 12 hours. Sampling, TLC shows that the reaction is complete, stop heating, cool naturally, until T 内 When the temperature is 70°C, add chlorobenzene (400mL), stir to make the system evenly mixed, add ice-water bath, and cool the reaction system to 0-5°C. 1M HCl aqueous solution (360 mL) was added dropwise, and the temperature in the reaction flask was up to 80°C. Separate the liquid and remove the upper chlorobenzene phase. 30% NaOH aqueous solution (700g) was added dropwise to the aqueous phase, then cyclohexane (800mL) was added for extraction, the layers were separated, and the cyclohexane phase was left, and the aqueous phase was extracted once more with cyclohexane (200mL). Separate the liquids, combine the organic phases, and wash the organ...
Embodiment 2
[0057] 1-[[2-(4-chlorophenyl)ethyl]amino]-2-chloropropane hydrochloride 100g, anhydrous AlCl 3 (1.5eq.) 75.2g was sequentially added into (500mL) four-neck flask, stirring was started, heated, and the temperature was raised to about 120°C for 12h. Sampling, TLC shows that the reaction is complete, stop heating, cool naturally, until T 内 Add tetrachloroethylene (400mL) when the temperature is 70°C, stir to make the system evenly mixed, add ice-water bath, and cool the reaction system to 0-5°C. 1M HCl aqueous solution (360 mL) was added dropwise, and the temperature in the reaction flask was up to 40°C. Separate the liquid and remove the lower tetrachloroethylene phase. 30% NaOH aqueous solution (700g) was added dropwise to the aqueous phase, then cyclohexane (800mL) was added for extraction, the layers were separated, and the cyclohexane phase was left, and the aqueous phase was extracted once more with cyclohexane (200mL). Separate the liquids, combine the organic phases, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com