Shakubiqu intermediate, Shakubiqu intermediate and preparation method of Shakubiqu
A technology of sacubitril and intermediate, which is applied in the field of preparation of sacubitril intermediate, sacubitril intermediate and sacubitril, can solve the problem of low total yield, difficult to effectively control isomer impurities, Dangerous and other problems, to achieve the effect of short synthesis route, improve total yield, and improve quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
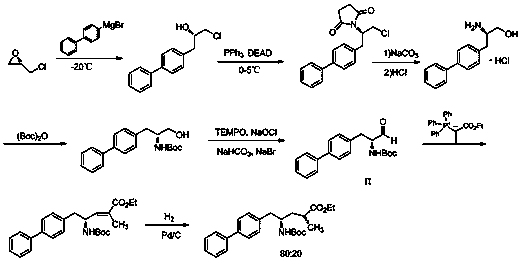
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of 3-carbonyl-5-biphenyl-pentanoic acid methyl ester (compound A, wherein R=methyl)
[0033] Add 38.6g of 4-cyanomethylbiphenyl, 300ml of THF and 20g of zinc powder into a 500ml reaction bottle, stir evenly, cool down to 5-10°C, drop a small amount of trimethylchlorosilane to initiate the reaction, and heat up to 50-60°C after adding ℃, add 35g methyl bromoacetate (X = Br) dropwise, after the dropwise addition, TLC detects that the raw materials basically disappear, cool down to 5-10℃, add 2M hydrochloric acid to adjust the pH value to 2-3, stir for 1 hour, add 200ml Ethyl acetate was separated, the aqueous layer was extracted with 300ml ethyl acetate, the combined organic layer was washed once with saturated brine 100ml, dried over anhydrous sodium sulfate, filtered, and concentrated to dryness under reduced pressure to obtain 48.2g of compound A, mol Yield 90.1%.
[0034] 1 H NMR (400 MHz, CDCl 3 ): δ 3.53(s, 2H), 3.74(d, 3H), 3.89(s, 2H),,7....
Embodiment 2
[0036]Example 2: Preparation of (R)-2-methyl-4-carbonyl-5-biphenylvalerate methyl ester (compound B)
[0037] Add 26.8g of the 3-carbonyl-5-biphenyl-valeric acid methyl ester obtained above, 200ml of freshly distilled anhydrous THF and 4.8g of sodium hydrogen (60% mineral oil solution) into a 500ml reaction bottle, and keep the temperature at 45-50 ℃, add 20.5g (R)-2-chloropropionic acid ethyl ester dropwise under stirring, continue to stir for 2 hours after dropping, drop to room temperature, then slowly add the reaction mixture dropwise to the ice-water mixture at 0~5℃ After the addition, add a mixture of 4.0 g sodium hydroxide and 10 g water dropwise under stirring, heat up to 10-15°C and continue stirring for 20 minutes, then add 2M hydrochloric acid to adjust the pH value to 2-3, and heat up to 30-30°C Stir at 35°C for 1 hour, add 200ml of ethyl acetate to separate layers, extract the aqueous layer twice with 200ml of ethyl acetate, wash the combined organic layer with 10...
Embodiment 3
[0040] Example 3: Preparation of (R)-2-methyl-4(R)-amino-5-biphenylpentanoic acid ethyl ester (compound C)
[0041] Add 300ml of methanol, 34.3g of isopropylamine, 700ml of water, and 120g of (R)-2-methyl-4-carbonyl-5-biphenylvalerate methyl ester (compound B) into a 2000ml reaction flask, and stir well. Add dropwise 1M hydrochloric acid to adjust the pH value to 8.7-9.2, add 6g transaminase and 0.6g coenzyme to control the pH value to 8.7-9.2, keep the reaction at 30-40°C for 12-20 hours, until the raw materials basically disappear as detected by TLC, and then reach 55-65 ℃ for 30 minutes, adding diatomaceous earth to filter, the filtrate was extracted with 600ml ethyl acetate, the extract was washed once with 160ml saturated saline and 180ml water respectively, then dried with anhydrous sodium sulfate, filtered, the filtrate was concentrated to dryness under reduced pressure, added 120g of methyl tert-butyl ether was heated and dissolved, cooled to 0-10°C to crystallize and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com