Fluorescence analysis method for measuring element content of lithium iron phosphate
A technology of lithium iron phosphate and fluorescence analysis, applied in measurement devices, analysis materials, material analysis using wave/particle radiation, etc., to achieve the effects of simple operation, reduced use, environmental pollution and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A fluorescence analysis method for determining the content of phosphorus and iron elements in lithium iron phosphate, comprising the following steps:
[0039] (1) Take the same batch of lithium iron phosphate samples and divide them into three equal parts;
[0040] (2) Add the first lithium iron phosphate sample to hydrochloric acid aqueous solution, heat to boil and keep for 18-25min, then cool to room temperature, and filter out unreacted carbon, and reduce the filtered solution with tin dichloride solution Ferric iron, then add CuSO 4 -Isatin indicator turns the light yellow solution into green, then add TiCl dropwise 3 After the green color of the solution disappears, add half a drop in excess, and the solution turns blue after standing; then add sulfuric acid / phosphoric acid mixed acid and sodium diphenylamine sulfonate indicator to form a solution to be tested; titrate it with potassium dichromate standard solution , when the solution changes from blue to purple...
Embodiment 2
[0046] 1. Potassium dichromate standard solution titration method to determine the content of iron in lithium iron phosphate samples:
[0047] Weigh 1.000g±0.005g (accurate to 0.0001g) lithium iron phosphate (sample 1), a positive electrode material in the lithium-ion battery industry, into a 100mL beaker, add (1+1) hydrochloric acid, and heat and boil on an electric heating furnace for 20 minutes Left and right, turn off the electric heating furnace, cool to room temperature, filter and set the volume to a 100mL volumetric flask for later use. Take 20mL of the above solution, add 30mL of water, 5mL (1+1) hydrochloric acid, put it on an electric heating furnace and heat to a slight boil, add 50g / L tin dichloride solution dropwise while it is hot until light yellow, add two drops of CuSO 4 -Isatin indicator turns green, add 20g / L TiCl dropwise 3 Solution until the green color disappears, half a drop in excess, and the solution turns blue when left to stand. Add 20mL of 15% s...
Embodiment 3-5
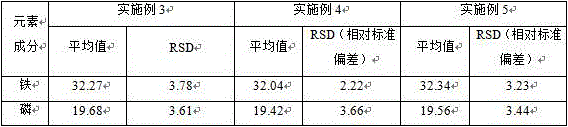
[0061] Select any batch of lithium iron phosphate samples, which are the positive electrode material of lithium-ion batteries, as samples 3-5, place them in molds such as aluminum rings with a diameter of 30mm, spread them evenly, press them with a little pressure, and then fill the entire mold with boric acid , after applying 20 tons of pressure on the tablet press for 5 seconds, it was pressed into a sheet, and the fluorescence intensity of iron and phosphorus in the sample to be tested was measured by X-ray fluorescence spectrometry, and the XRF standard curve was drawn using Example 2 and Determination of iron and phosphorus content.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com