2,4-diamine-1,3,5-triazine compound, preparation method and application thereof
A compound and triazine technology, applied in the field of 2,4-diamine-1,3,5-triazine compounds, can solve the problems of difficult control of reaction conditions, complicated steps, poor yield, etc. Industrial application prospects, easy operation, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
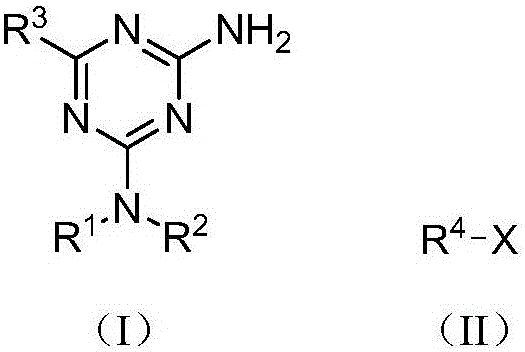
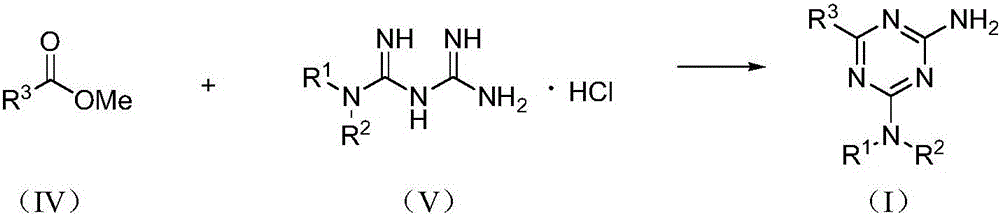
[0028] Example 1 Preparation of 2-amino-1,3,5-triazine compound (I)
[0029] Taking 2-amino-4-dimethylamino-6-phenyl-1,3,5-triazine as an example, its preparation method is:
[0030] Mix methyl benzoate (react IV, 0.1362 g, 1 mmol), metformin hydrochloride (react V, 0.0662 g, 0.4 mmol), sodium methoxide (0.0540 g, 1 mmol) in methanol (10 mL), and Reaction under the condition of (25°C), TLC tracking monitoring, reaction for 12h, after that, the reaction solution was evaporated to remove the solvent, 10mL of water was added, filtered, and the filter cake was recrystallized with methanol to obtain white crystals, which were dried to obtain 2-amino-4-dimethylamine 0.0563 g of phenyl-6-phenyl-1,3,5-triazine, yield 65.4%.
Embodiment 2~9
[0031] Examples 2-9 Preparation of 2-amino-1,3,5-triazine compound (I)
[0032] The following Examples 2-9 were prepared according to the method described in Example 1, wherein different reaction substrates and their feeding amounts, different reaction products and their yields are listed in Table 1.
[0033] Reaction substrate and its charging amount in the embodiment 2~9 of table 1, reaction product and its productive rate
[0034]
Embodiment 10
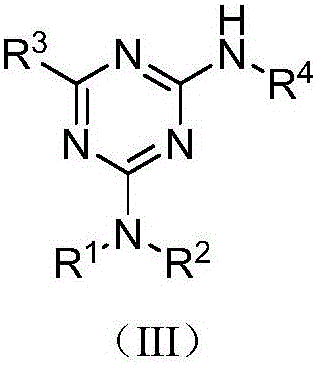
[0035] Embodiment 10: the preparation of compound (III-1)
[0036] Add 2-amino-4-dimethylamino-6-phenyl-1,3,5-triazine (0.1076g, 0.50mmol), p-methoxy iodobenzene (0.1170g, 0.50mmol) into the reaction vessel , cuprous iodide (0.0285g, 0.15mmol), potassium carbonate (0.1380g, 1.00mmol), N,N'-dimethylethylenediamine (DMEDA, 0.0396g, 0.45mmol) and solvent acetonitrile (3mL), Reflux for 10 hours. Cool after the reaction, add ammonia water 4mL, stir for 5 minutes, add saturated NaCl aqueous solution (20mL), extract with ethyl acetate (20mL×3), combine organic layers, concentrate, column chromatography (eluent is petroleum ether: Ethyl acetate=5:1, v:v), collect R f The eluate with a value of 0.4-0.45 (monitored by TLC, the developing solvent is the same as the eluent), the solvent was distilled off under reduced pressure, and dried to obtain 0.1222 g of the target compound (III-1), with a yield of 76.0%. 1 H NMR (500MHz, CDCl 3 ):δ8.44-8.42(m,2H),7.58(d,J=9.0Hz,2H),7.53-7.46(m,3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com