A kind of β-mannanase mrmman5a and its coding gene and application
A technology for encoding genes and amino acids, applied to β-mannanase mRmMan5 and its encoding genes and application fields, can solve the problems of few reports and other problems, and achieve the effects of high optimum temperature, high-efficiency expression, and great application value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1, the directed evolution of β-mannanase gene
[0049] 1. Random mutation of β-mannanase gene (RmMan5A)
[0050] 1. Error-prone PCR
[0051] Sequence analysis of the wild-type gene of Rhizomucor miehei β-mannanase (as shown in Sequence 2 of the Sequence Listing) revealed that the 5' end of the gene contains a sequence encoding a signal peptide consisting of 19 amino acid residues. Error-prone PCR primers RmMan5AF and RmMan5AR were designed according to the mature protein coding region of Rhizomucor miehei β-mannanase (that is, the signal peptide coding sequence was removed). The primer sequences are as follows:
[0052] RmMan5AF: 5'-CGC GGATCC GCTTCTTCGTTTGTCCAGACAAG-3'; the underlined sequence is the recognition site for BamHI digestion; the underlined sequence is identical to the 58th to 80th sequence of sequence 2 or 4 in the sequence listing;
[0053] RmMan5AR: 5'-CCG CTCGAG TCACTTCTTGGCCATGGCATCAGC-3'; the underlined sequence is the XhoI restricti...
Embodiment 2
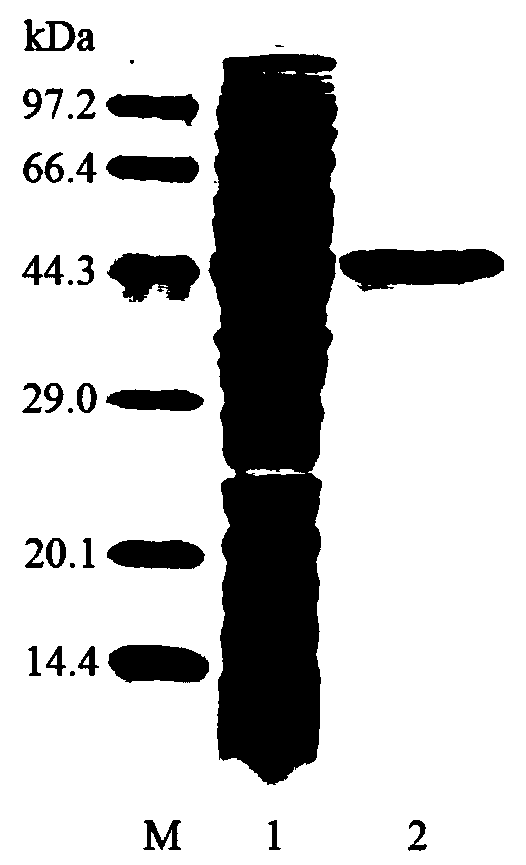
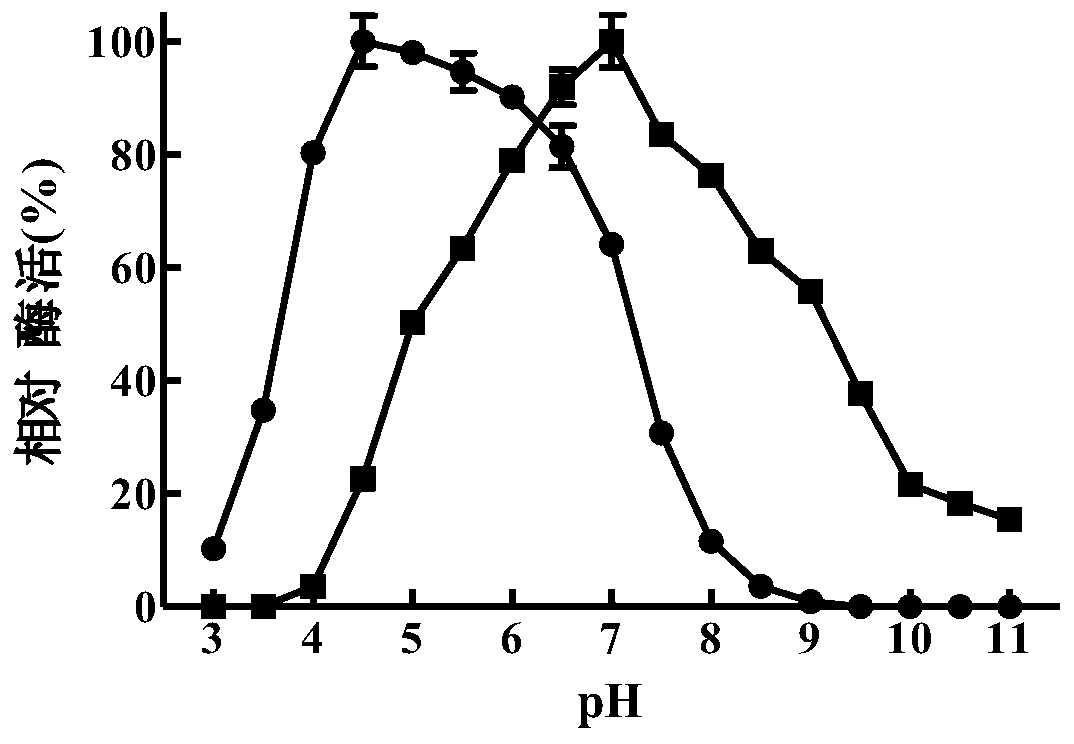
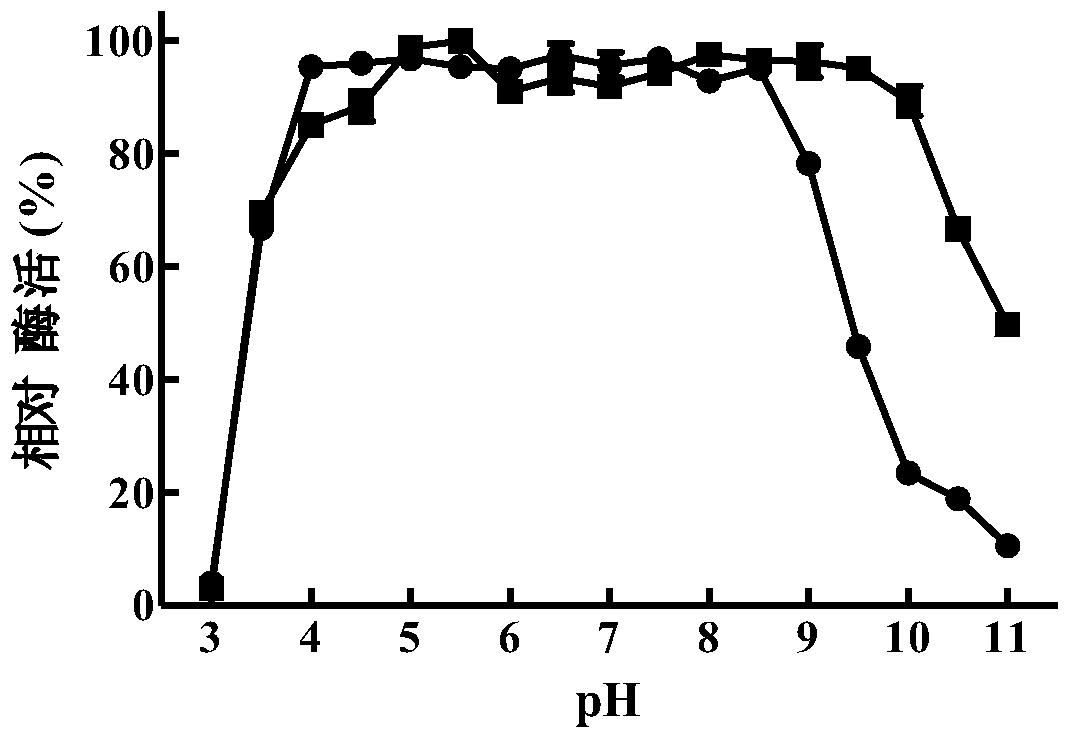
[0083] Embodiment 2, preparation of β-mannanase and determination of enzymatic properties thereof
[0084] 1. Preparation of recombinant protein
[0085] 1. Induced expression of recombinant protein
[0086] Inoculate recombinant bacteria A or control bacteria into liquid LB medium (containing 50 μg / mL kanamycin) and shake culture (37°C, 200rpm), until OD 600 When the concentration reaches 0.6-0.8, add IPTG to a final concentration of 1 mmol / L, induce culture at 30°C overnight, collect the bacteria at 10,000 × g, resuspend, ultrasonically break, centrifuge at 10,000 × g for 10 min, and collect the supernatant as the crude enzyme solution .
[0087] 2. Purification of recombinant protein
[0088] Recombinant proteins were purified using Sepharose Ni-IDA affinity columns. Specific steps are as follows:
[0089] Use buffer A to equilibrate the Ni-IDA affinity column for 5-10 column volumes, load the crude enzyme solution of recombinant bacteria A or control bacteria obtained...
Embodiment 3
[0118] Example 3, Pichia pastoris high-density fermentation expression of recombinant protein mRmMan5A
[0119] 1. Acquisition of recombinant bacteria
[0120]1. Using the extracted plasmid (containing the mRmMan5A gene) obtained in Example 1 as a template, PCR amplification was performed using mRmMan5AF and mRmMan5AR primer pairs to obtain a PCR amplification product. The primer sequences are as follows:
[0121] mRmMan5AF: 5'-CCATG TACGTA GCTTCTTCGTTTGTCCAGACAAG-3'; the underlined sequence is the SnaBI restriction recognition site; the underlined sequence is identical to the 58th to 80th sequence of sequence 2 or 4 in the sequence listing;
[0122] mRmMan5AR: 5'-CCG CCTAGG TCACTTCTTGGCCATGGCATC-3'; the underlined sequence is the AvrII restriction recognition site; the underlined sequence matches the 1117th to 1137th sequence of sequence 2 or 4 in the sequence listing.
[0123] 2. Carry out double enzyme digestion to the PCR amplified product obtained in step 1 with res...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com