A pullulanase mutant
A technology of pullulanase and mutants, which is applied in the field of combined site-directed mutation of pullulanase enzyme molecules, screening and expression preparation of mutants, and can solve the problem of not being able to play a good role in starch debranching and increasing pullulanase Problems such as the amount of usage and the narrow range of optimal action conditions achieve the effect of high-efficiency and low-cost fermentation preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] Example 1: Selection of pullulanase mutation sites
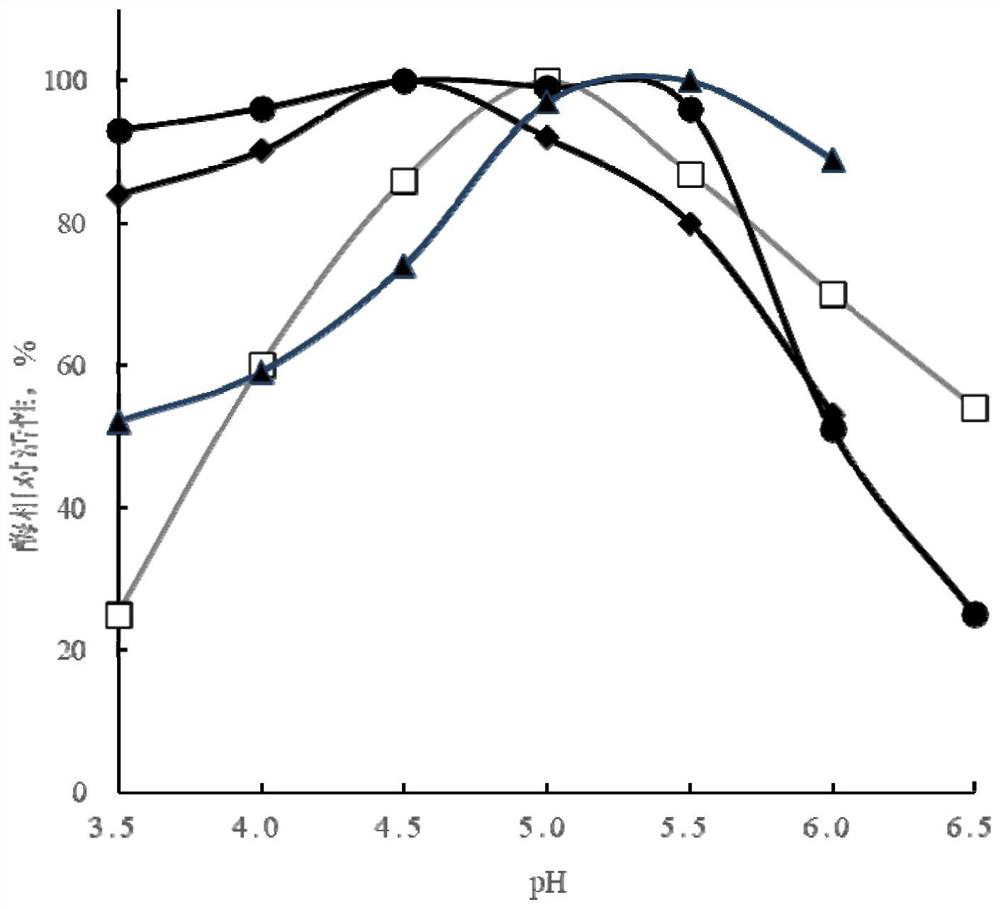
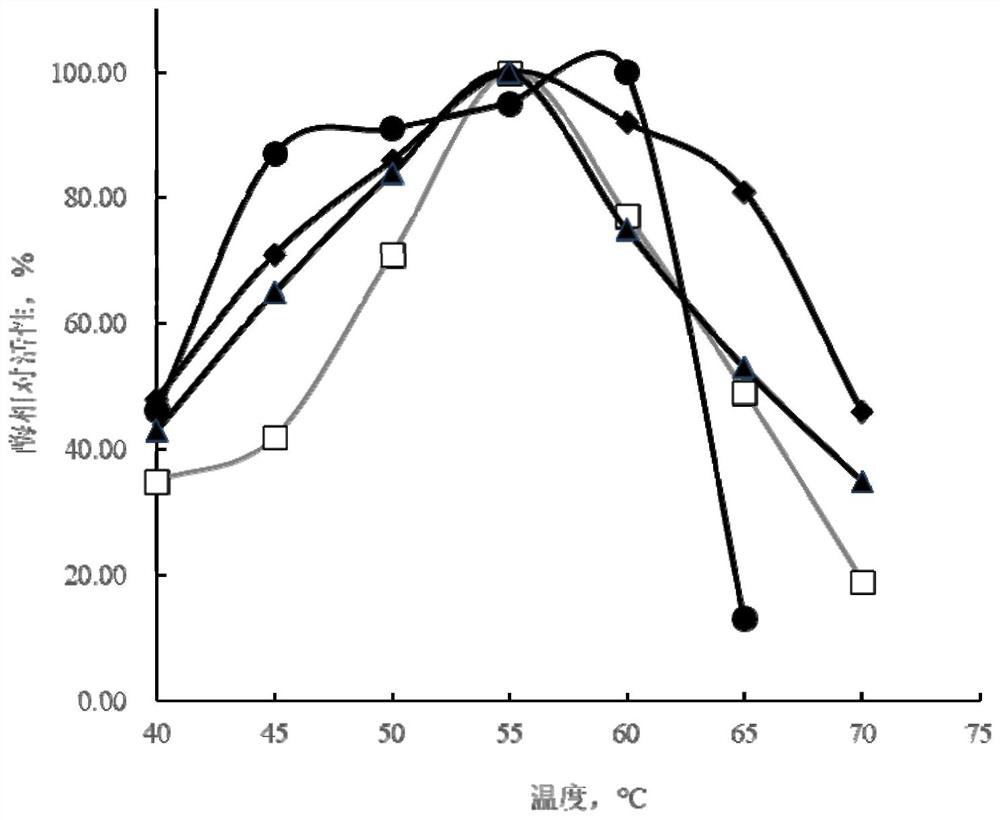
[0168] The present invention selects the mutants according to the following criteria: 1) the optimal temperature of the mutant is increased to 60°C or above, and the enzyme activity is not lower than that of PulA; 2) the optimal temperature of the mutant is reduced to 50°C or below , and the enzyme activity was not lower than that of PulA; 3) The optimum pH of the mutant was reduced to 4.5 or below, and the enzyme activity was not lower than that of PulA; 4) The optimum pH of the mutant was increased to 5.5 or above, and the enzyme activity Compared with PulA, there is no decrease; 5) The enzyme activity of the mutant is at least 10% higher than that of the wild-type pullulanase. Taking one of the above selection criteria as the selection criteria, 54 amino acid residues were screened and obtained, namely S99, E100, Q108, S112, A146, A235, A272, N317, T238, N322, K327, N342, A347, T355 , A356, S357, G358, T385, A414,...
Embodiment 2
[0169] Example 2: Pullulanase combinatorial mutants with beneficial changes in enzymatic properties and catalytic efficiency
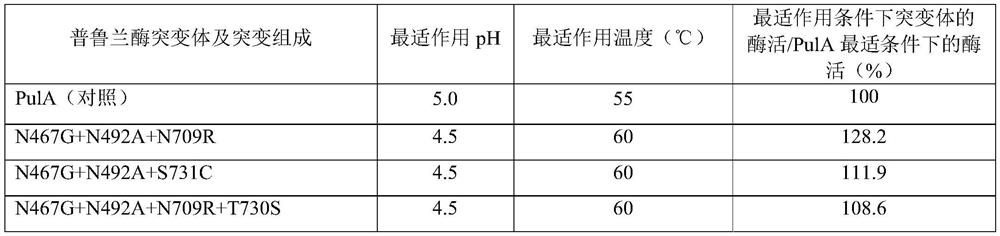
[0170] Based on the 54 amino acid residue sites obtained in Example 1, combined site-directed mutagenesis was performed, and the mutated coding gene was expressed in Bacillus licheniformis strain M208236, and the pullulanase mutant was prepared and purified, and then analyzed and determined. The optimum temperature, optimum pH, specific enzyme activity, etc. of the mutants were compared with those of the wild-type pullulanase.
[0171] (1) After the combination mutation of pullulanase, the combination of the following mutation sites is conducive to the debranching and hydrolysis of starch by the pullulanase mutant at pH 4.5 and / or 60 °C, and at its optimum pH and The enzyme activity at the optimum temperature was not lower than that of the wild-type PulA at its optimum pH and temperature. E.g:
[0172] N467G+N492A+N709R
[0173] N467G+N492A+S731C
...
Embodiment 3
[0229] Example 3: Fermentative preparation of pullulanase mutants
[0230] The pullulanase mutant obtained in Example 2, the combined mutant N467G+N492A+N709R (nucleotide sequence SEQ ID NO.3, amino acid sequence SEQ ID NO.4), combined mutant N467G+N492A +A591S+N709R+G723S (nucleotide sequence SEQ ID NO. 5, amino acid sequence SEQ ID NO. 6) and combined mutants N467G+N492A+N709R+G723S+S731C (nucleotide sequence SEQ ID NO. 7, amino acid sequence SEQ ID NO. 7) Sequence SEQ ID NO.8), cloned into the expression vector pHY-WZX, genetically transformed into the Bacillus licheniformis strain CCTCC NO.M208236 to obtain a pullulanase-producing recombinant bacterium, and its corresponding recombinant bacterium was renamed respectively: Pu13M002 , Pul5M004 and Pul5M006. Fermentation in 30L fermentation tank for 96h (fermentation conditions: fermentation medium consists of: maltose syrup 2%, cottonseed flour 2%, bean cake flour 2%, ammonium sulfate 0.5%, the rest are water, pH 7.0; the i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com