HPV16E7 monoclonal antibody and preparation method and application thereof
A HPV16E7, monoclonal antibody technology, applied in the direction of antibodies, chemical instruments and methods, anti-tumor drugs, etc., can solve the problems of high false-positive rate of monitoring results and difficult implementation in small hospitals, and achieve high application value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The present invention provides a preparation method of HPV16E7 monoclonal antibody, comprising the following steps,
[0036] Step 1, HPV16E7 gene amplification
[0037] Using HPV16 positive secretion as a template for PCR amplification, the upstream primer sequence is shown in SEQNO.1, and the downstream primer sequence is shown in SEQNO.2;
[0038] Step 2, obtaining HPV16E7 recombinant protein
[0039] Using the PCR product obtained in recovery step 1 to carry out HPV16E7 expression vector construction and induced expression, and carry out the purification of HPV16E7 recombinant protein;
[0040] Step three, immunization
[0041] The antigen used is the purified HPV16E7 recombinant protein obtained in step 2;
[0042] Step 4, cell fusion to obtain hybridoma cells;
[0043] Step 5, screening positive clones of the hybridoma cells obtained in step 4 to obtain specific HPV16E7 monoclonal antibody hybridoma cells;
[0044] Step six, using the specific HPV16E7 monoclonal...
Embodiment 1
[0046] The preparation of embodiment one HPV16E7 antigen
[0047] First, design and synthesize HPV16E7 primers, and design a pair of specific primers based on the HPV16E7 gene sequence released by NCBI. Upstream primer (SEQNO.1): 5'-CGC GGATCC ATGCATGGAGATACACCTACATTG' (the underlined part is the BamHI restriction site); downstream primer (SEQNO.2): 5'-CCG CTCGAG CTATTATGGTTTCTGAGAACAGATG-3' (the underlined part is the XhoI restriction site).
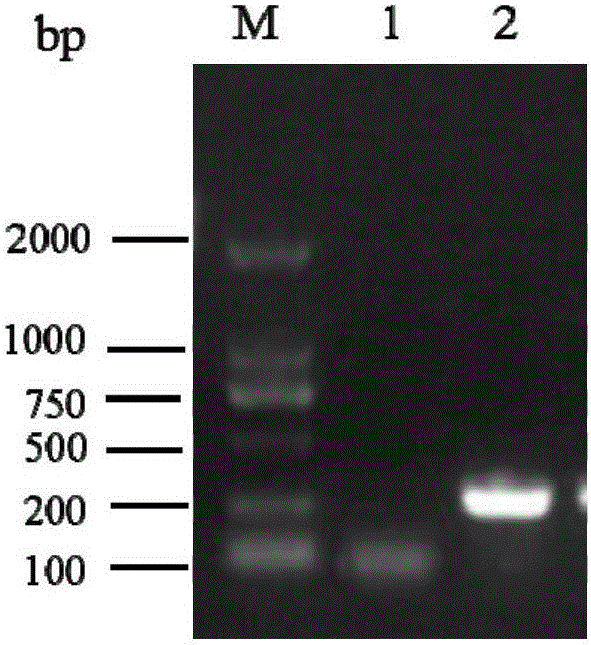
[0048] Then, the HPV16E7 gene was amplified, and the HPV16 positive secretion was used as a template for PCR amplification. The reaction system is as follows: 10×PCR buffer 2μl, MgCL 2 (25mmol / L) 1.5ul, dNTP0.4μl, upstream and downstream primers 0.5μl, template DNA 1μl, Taq enzyme 0.2μl, deionized water 13.9μl; reaction conditions: 94°C for 5min, 94°C, 45s→56°C, 45s→72°C, 45s, 30 cycles, 72°C extension for 7min, the results are attached figure 1 As shown (Note: M: marker; 1: negative specimen; 2: HPV16 positive specimen).
[004...
Embodiment 2
[0051] The preparation of embodiment secondary anti-HPV16E7 monoclonal antibody
[0052] 1) Immunization of mice Eight-week-old Balb / C mice were immunized with the purified HPV16E7 antigen in Example 1. The way of immunization is multi-point subcutaneous injection, the immunization dose is 0.05 mg / monkey, and the immunization interval is 2 weeks. Complete Freund's complete adjuvant was added for the first immunization, followed by three times of immunization with Freund's incomplete adjuvant, and finally the antigen solution was used as a shock immunization.
[0053] 2) Cell fusion and hybridoma cell selection After 3 days of shock immunization, mouse splenocytes were taken to fuse with SP2 / 0 cells, and HAT was used to select culture at 37°C, 5% CO 2 cultured in an incubator. After 10 days, use HPV16E7 as the antigen-coated ELISA plate (10ng / well), detect the culture supernatant and screen the positive clones by indirect ELISA, and continue to limit the dilution of the scree...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com