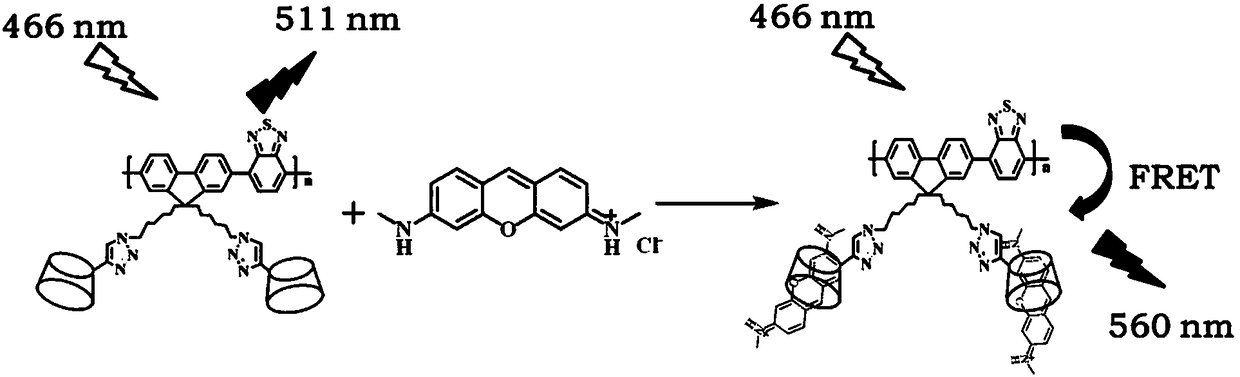
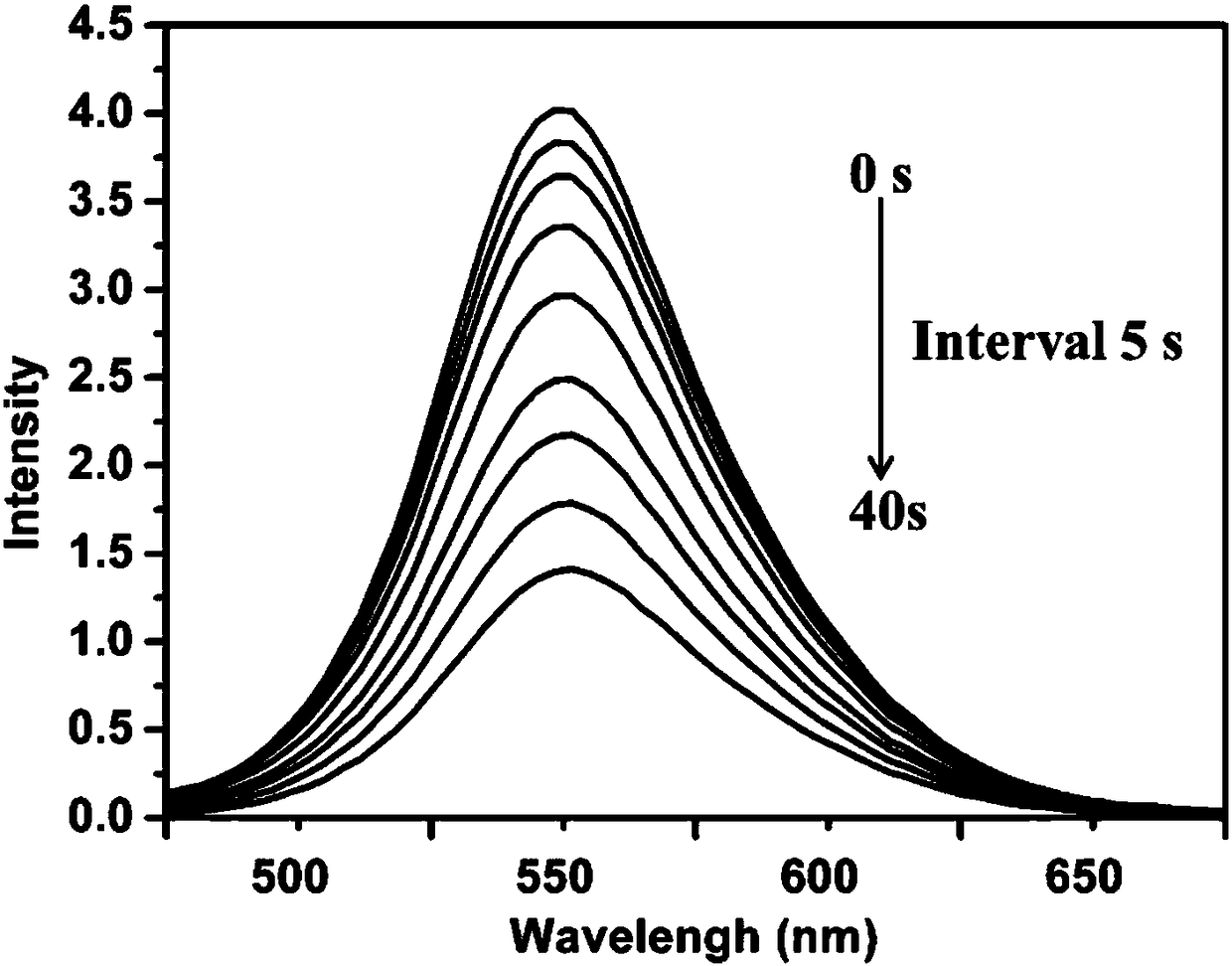
Fluorene conjugated polymer film based on cyclodextrin side chain and its preparation method and application
A conjugated polymer, cyclodextrin technology, applied in fluorescence/phosphorescence, material excitation analysis, etc., can solve the problems of cumbersome synthesis methods, limited wide use, etc., to improve the emission wavelength, increase the Stokes shift, and simplify complex methods. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The preparation method of the cyclodextrin side chain i.e. propargyl-cyclodextrin is as follows:
[0052] Disperse the cyclodextrin (3.2-12.8 mmol) in water, and slowly add an aqueous solution of sodium hydroxide (3.2-12.8 mmol) dropwise in an ice-water bath to completely dissolve the cyclodextrin. At this time, p-toluenesulfonyl chloride (4.6-18.4 mmol) was dissolved in acetonitrile solution and slowly added dropwise to the above-mentioned clear liquid, during which white precipitates were formed. After the mixture was reacted at room temperature for 4 h, the pH value was adjusted to 6 with 1-3 mol / L saline solution, filtered, and recrystallized in water to obtain the target product. Dissolve the obtained product mono-6-deoxy-6-p-toluenesulfonyl-cyclodextrin (1~3mmol) and propargylamine (2~6mmol) in DMF, and react at 100~110°C for 4h under nitrogen protection . After the reaction, the solution was cooled to room temperature and settled in acetone. The solid was colle...
Embodiment 1
[0055] The main chain of the fluorene conjugated polymer, that is, the end group of the side chain is -N 3 The preparation method of the fluorene conjugated polymer comprises the steps:
[0056] (1) 1mmol of 2,7-dibromofluorene is added to 50mL of mass fraction in 50% NaOH aqueous solution, then 1mmol of phase transfer catalyst tetrabutylammonium bromide, 10mmol of 1,6-dibromohexane is added, After reacting at 75°C for 4h, cool to room temperature, pass CH 2 Cl 2 Extraction, and then washed with 0.5mL dilute hydrochloric acid aqueous solution and water, anhydrous MgSO 4 Drying, followed by silica gel column purification, eluent is n-hexane / dichloromethane (volume ratio 10:1), to obtain white solid 9,9'-bis(bromohexyl)-2,7-dibromofluorene, this step The yield was 52%.
[0057] (2) 5 mmol of compound 9,9'-di(bromohexyl)-2,7-dibromofluorene, 15 mmol of NaN 3 Dissolve in 35 mL of DMSO, react for 4 h under nitrogen protection, 75 °C, cool to room temperature, add 150 mL of wat...
Embodiment 2
[0064] The main chain of the fluorene conjugated polymer, that is, the end group of the side chain is -N 3 The preparation method of the fluorene conjugated polymer comprises the steps:
[0065] (1) 1mmol of 2,7-dibromofluorene is added to 50mL of mass fraction in 50% NaOH aqueous solution, then 1mmol of phase transfer catalyst tetrabutylammonium bromide, 10mmol of 1,6-dibromohexane is added, After reacting at 75°C for 4h, cool to room temperature, pass CH 2 Cl 2 Extraction, and then washed with 0.5mL dilute hydrochloric acid aqueous solution and water, anhydrous MgSO 4 Drying, followed by silica gel column purification, eluent is n-hexane / dichloromethane (volume ratio 10:1), to obtain white solid 9,9'-bis(bromohexyl)-2,7-dibromofluorene, this step The yield was 52%.
[0066] (2) 5 mmol of compound 9,9'-di(bromohexyl)-2,7-dibromofluorene, 15 mmol of NaN 3 Dissolve in 35 mL of DMSO, react for 4 h under nitrogen protection, 75 °C, cool to room temperature, add 150 mL of wat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com