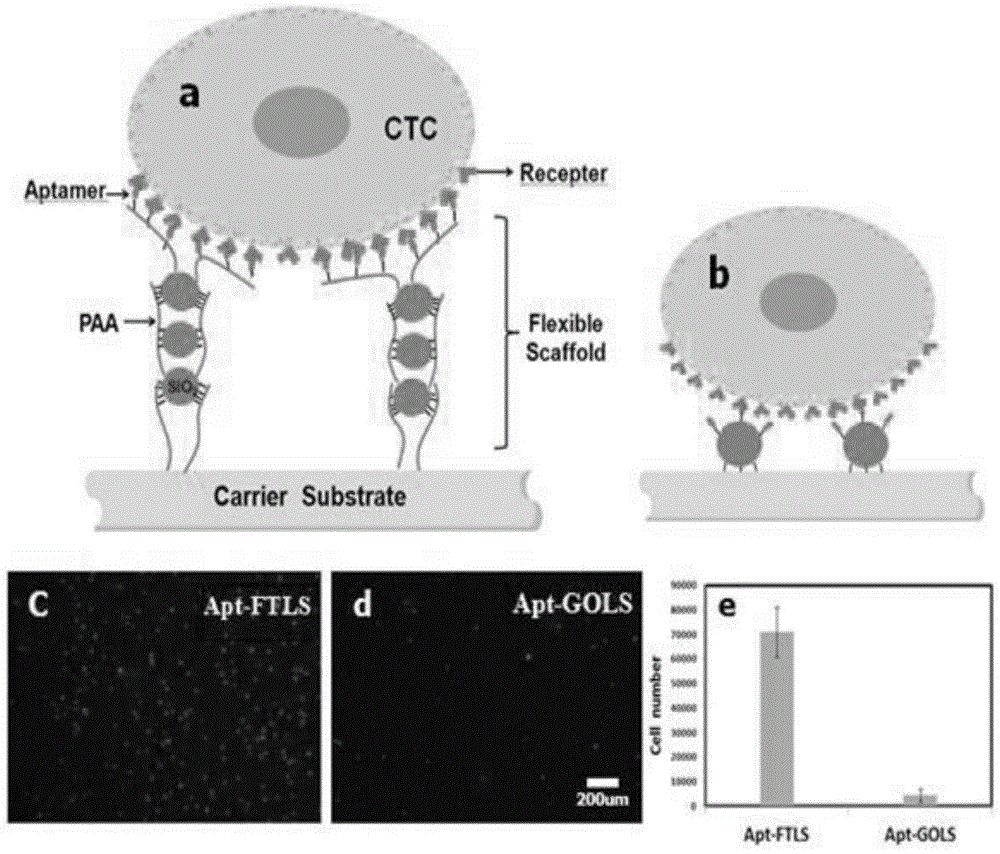
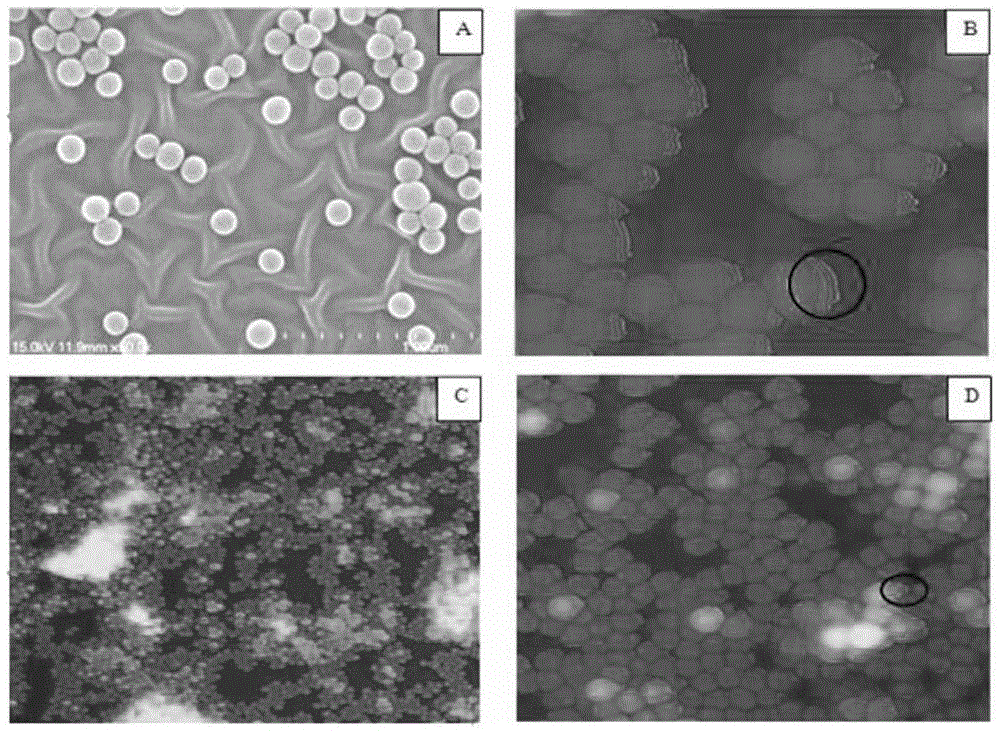
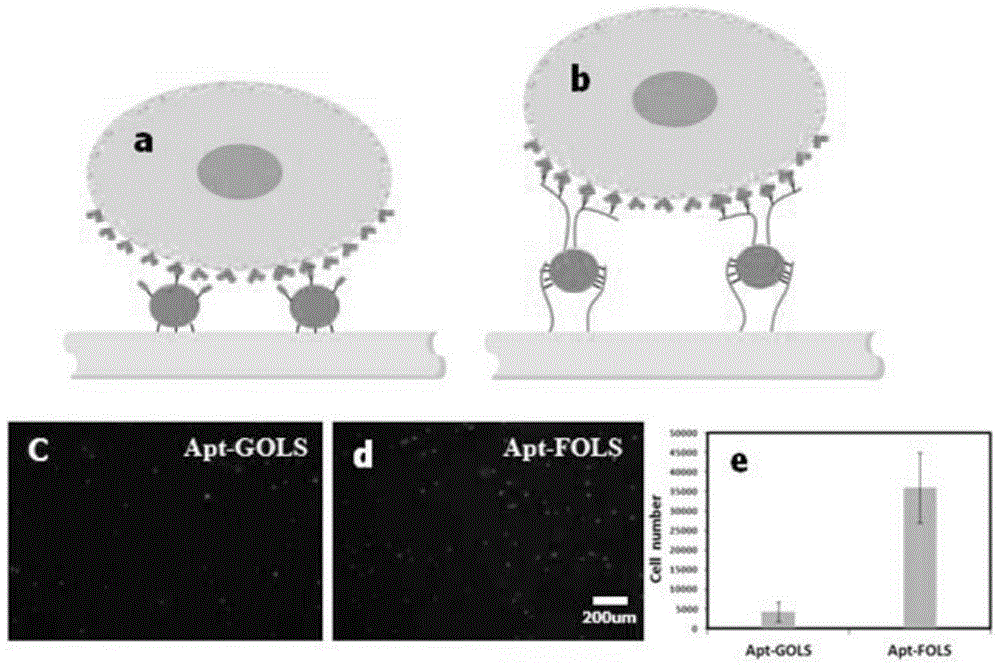
Multi-capture-ligand-modified multi-layer nanoparticle flexible stent of target cell and application of multi-layer nanoparticle flexible stent
A technology of target cells and nanoparticles, which is applied in the field of multi-layered nanoparticle flexible scaffolds, can solve problems such as the inability to capture CTCs, and achieve the effects of high specific capture efficiency, high roughness, and low non-specific adsorption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0031] Specific embodiment 1: SiO 2 Preparation of nanoparticles and their silanization
[0032] Add 25 mL of absolute ethanol at constant temperature to 40°C, 1.5 mL of ammonia water, and 0.5 mL of water into the two-neck flask in turn, and quickly add 0.75 mLTEOS (tetraethyl orthosilicate) in a constant temperature water bath at 40°C under stirring at 500 rpm / min. 7h, then add 0.5mLTEOS, continue to react for 15h, stop the reaction. Centrifuge at high speed, discard the supernatant, wash the precipitate with absolute ethanol and three-distilled water in turn, and finally redissolve in 25mL three-distilled water for later use to obtain SiO 2 Nanoparticle solution (11 mg / mL).
[0033] A silanized hydrolyzate was prepared by stirring with 19 mL of absolute ethanol, 0.3 mL of APTES (3-aminopropyltriethoxysilane) and 0.3 mL of triple-distilled deionized water for 5 min. Take 11mg / mLSiO 2 2 mL of nanoparticle solution, centrifuged for 20 min, redissolved in 1 mL of absolute et...
specific Embodiment 2
[0034] Specific Example 2: Preparation of flexible nanoparticle scaffold and connection of its aptamer
[0035] Preparation of silanized carrier matrix: 30% hydrogen peroxide and 98% sulfuric acid were mixed at a volume ratio of 3:7 to prepare a lotion. Cut the slide into 25.4×19mm 2 sized rectangular glass slides as carrier substrates. Wash the carrier matrix with water and absolute ethanol respectively, blow dry, put into the lotion, heat at 80-100°C for 1 hour, cool naturally, wash the carrier matrix with triple-distilled deionized water, and blow dry with cold wind. The carrier matrix was reacted in 2% APTES absolute ethanol solution for 18 hours, rinsed with triple distilled deionized water, dried, and baked at 120°C for 6 hours to obtain a silanized carrier matrix with surface-coupled amino groups.
[0036] Preparation of activated PAA solution: Take 100 μL of polyacrylic acid (PAA, 25wt% aqueous solution, Mw=240000) in a 10mL single-necked bottle, add 5mL of three-dis...
specific Embodiment 3
[0039] Specific Example 3: Cell Culture and Cell Capture
[0040] Cell culture and staining: For Ramos and HL-60 cells, in the prepared culture solution (the culture solution was prepared with RPMI-1640 medium, in which FBS contained 15%, and philin-streptomycin was 100 U / mL), 37 5% CO at °C 2 Cultivate in saturated humidity. Ramos cells were labeled with orange-red DiI dye, while HL-60 cells were labeled with green DiO dye. Suspend the cells in serum-free medium, the corresponding cell density is 1.0×10 6 cells / mL, add 5 μL of cell labeling solution to every 1 mL of cell suspension, mix well, incubate at 37°C for 10-20 minutes, centrifuge at 1500 rpm for 5 minutes, and gently resuspend the cells with 37°C medium. Repeat 2 washes with 5 mL of Wash Buffer WB, and finally suspend it in 6 mL of BB to obtain 1.0×10 6 cells / mL of stained cell suspension. Wherein DiI and DiO solutions adopt DMSO as the solvent, respectively prepare solutions containing DiI and DiO dyes at a con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com