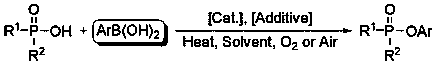A kind of method for preparing phosphinic acid/phosphinic acid/phosphate ester with p(o)-oh compound and arylboronic acid
A technology of phosphinic acid and phosphinic acid is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., and can solve problems such as cumbersome experimental steps, harsh reaction conditions, and environmental pollution. To achieve the effect of simple and easy method, simple preparation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 218 mg (1.0 mmol) of diphenylphosphoric acid, 122 mg (1.0 mmol) of phenylboronic acid, 120 mg (2.0 mmol) of urea, 100 mg of molecular sieve and 28.7 mg (0.2 mmol) of cuprous bromide under oxygen atmosphere In a Schlenk tube, add 3.0 ml of organic solvent (tetrahydrofuran, diethyl ether, toluene, 1,4-dioxane, N,N -dimethylformamide, dimethyl sulfoxide, acetonitrile), at 80 o The reaction was stirred at C for 12 hours. Through GC detection and analysis, when acetonitrile is used as the reaction solvent, the productive rate of the coupling reaction can reach 92% productive rate.
Embodiment 2
[0027] Add 218 mg (1.0 mmol) of diphenylphosphoric acid, 122 mg (1.0 mmol) of phenylboronic acid, 2.0 mmol of base (triethylamine, sodium bicarbonate, potassium carbonate, sodium carbonate, urea, thiourea, cesium carbonate, or phosphoric acid Potassium), 100 mg molecular sieves and 28.7 mg (0.2 mmol) cuprous bromide were added to the Schlenk tube under oxygen atmosphere, and 3.0 ml acetonitrile was added under oxygen atmosphere, and at 80 o The reaction was stirred at C for 12 hours. Through GC detection and analysis, when acetonitrile is used as the reaction solvent, the productive rate of the coupling reaction can reach 92% productive rate.
Embodiment 3
[0029] Add 218 mg (1.0 mmol) of diphenylphosphoric acid, 122 mg (1.0 mmol) of phenylboronic acid, 120 mg (2.0 mmol) of urea, 100 mg of additives (molecular sieves, phosphorus pentoxide, anhydrous sodium sulfate, anhydrous Magnesium sulfate, anhydrous calcium chloride) and 28.7 mg (0.2 mmol) of cuprous bromide were added to the Schlenk tube under oxygen atmosphere, and 3.0 ml of acetonitrile was added under oxygen atmosphere, at 80 o The reaction was stirred at C for 12 hours. Through GC detection and analysis, when acetonitrile is used as the reaction solvent, the productive rate of the coupling reaction can reach 92% productive rate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com