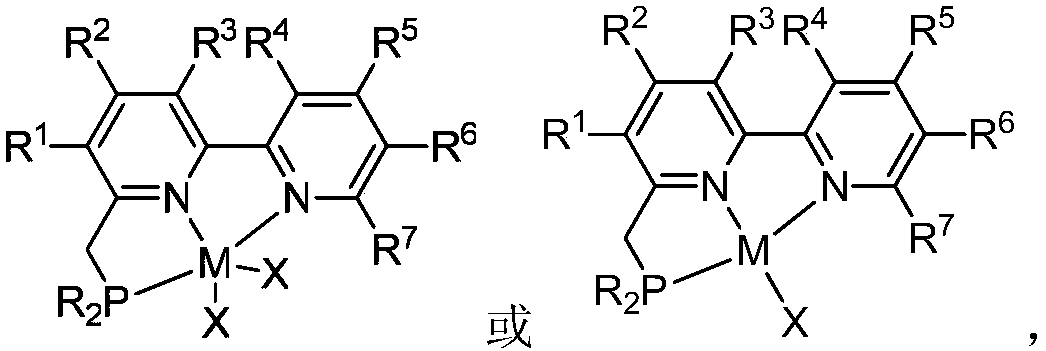
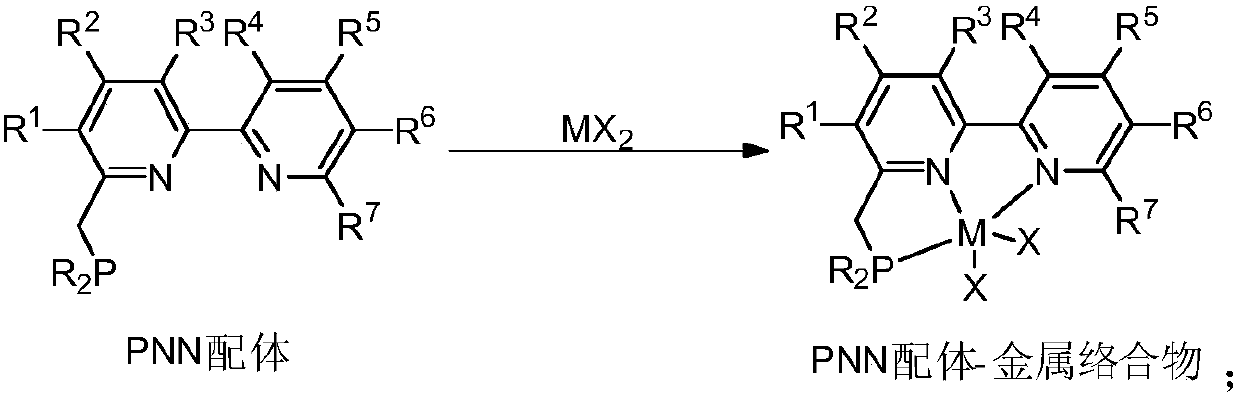
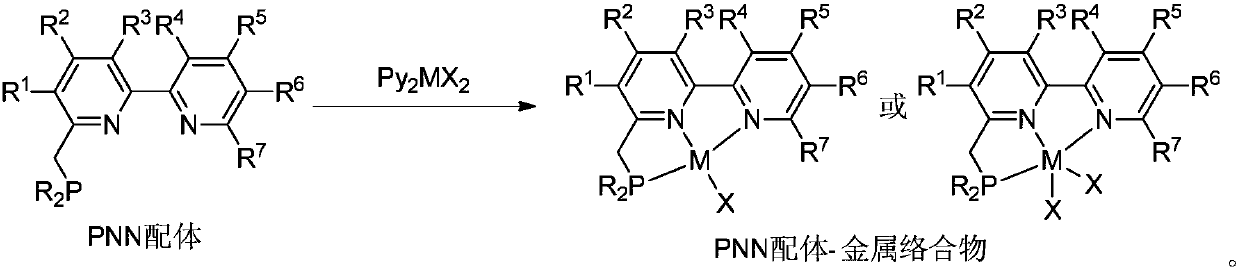
A kind of pnn ligand-cobalt complex catalyst and its preparation method and application
A complex and catalyst technology, applied in organic compound/hydride/coordination complex catalysts, organic compound preparation, physical/chemical process catalysts, etc., can solve problems such as poor regioselectivity, achieve high yield, The raw materials are cheap and easy to obtain, and the reaction conditions are mild.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: prepare PNN ligand-cobalt complex
[0041] ( t Bu-PNN)CoCl 2 (Complex A):
[0042] In the glove box, the CoCl 2 (260mg, 2.0mmol, 1.0equiv) and THF (50mL) were added to a 100mL schlenk tube, and then slowly t A THF solution (10 mL) of Bu-PNN ligand (628 mg, 2.0 mmol, 1.0 equiv) was added dropwise to the above solution, and the color of the reaction solution gradually turned black. After the reaction was stirred at room temperature for 24 h, the reaction solution was concentrated to 10 mL with an oil pump, and then Et 2 O, when the solid precipitated out, it was filtered and washed with ether, and the solvent was drained to obtain a purple powder (826mg, 93%). Then the above powder (50 mg) was dissolved in CH 2 Cl 2 (3mL), add 1mL CH 2 Cl 2 A mixed solvent (1:1) with n-hexane is used as a buffer layer, and a large amount of n-hexane is added to the upper layer of the mixed solvent, and it is left to stand for several days until n-hexane slowly dif...
Embodiment 2
[0049] Embodiment 2: the catalytic activity experiment of complex B described in embodiment 1 to the hydroboration reaction of different single olefins
[0050]
[0051] Taking the hydroboration process of olefin 1a as an example: firstly, in the glove box, complex B (2.1mg), THF (20mL) and NaBEt 3 H (1M) (10uL) was added into a 50ml Erlenmeyer flask to obtain a purple catalyst solution; then olefin 1a (63mg, 0.5mmol, 1equiv) and HBpin (75uL, 0.5mmol, 1equiv) were added to an 8mL reaction vial, and then 1mL of the prepared catalyst solution was added into the above-mentioned reaction vial; after the reaction was stirred at room temperature for 15min, it was exposed to the air and quenched; mixture as eluent) to obtain colorless liquid 3a. The preparation method of 3b~3n, 4b~4m is the same as the preparation method of 3a.
[0052] 4,4,5,5-tetramethyl-2-nonyl-1,3,2-dioxaborolane (3a):
[0053] Colorless liquid (121.0mg, 95%); 1 H NMR (400MHz, CDCl 3 )δ=1.35-1.43(m,2H,C...
Embodiment 3
[0096] Example 3: Hydroboration of olefins carried out under solvent-free conditions
[0097]
[0098] In the glove box, alkene 2a (5.2g, 50mmol) and HBpin (6.4g, 50mmol, 1equiv), complex B (1.0mg) and NaBEt 3 H(1M) (5 uL) was added to an 8 mL reaction vial. After the reaction was stirred at room temperature for 1 h, it was quenched by exposure to air. Flash column chromatography (silica gel with a height of about 5 cm, a mixture of petroleum ether and ethyl acetate as eluent) gave colorless liquid 4a (m=11.5 g, yield 99%).
[0099] 4,4,5,5-tetramethyl-2-phenethyl-1,3,2-dioxaborolane (4a):
[0100] Colorless liquid (106.0mg, 91%); 1 H NMR (400MHz, CDCl 3 )δ=7.34-7.24(m,4H,aryl-H),7.23-7.16(m,1H,aryl-H),2.80(t,J=8.0Hz,2H,PhCH 2 ),1.26(s,12H,C(CH 3 ) 2), 1.20(t, J=8.0Hz, 2H, BCH 2 ). 13 C NMR (101MHz, CDCl 3 )δ144.5(aryl-C), 128.3(aryl-C), 128.1(aryl-C), 125.6(aryl-C), 83.2(OC(CH 3 ) 2 ), 30.1 (PhCH 2 ),24.9(C(CH 3 ) 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com