Novel peptide tag and uses thereof
A technology of peptide tags and polynucleotides, applied in the field of epitope labeling systems, can solve the problems of reducing test reliability, hindering protein separation and confirmation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] For example, one example of the present invention provides a method for preparing the above-mentioned fusion protein. A method for preparing a fusion protein according to an example of the present invention includes: culturing a transformant introduced with a polynucleotide encoding a fusion protein to express the fusion protein. In this case, the polynucleotide encoding the fusion protein is preferably introduced into the transformant in the form of a recombinant vector. In addition, the above-mentioned recombinant vector includes: a polynucleotide encoding the above-mentioned peptide tag and a polynucleotide encoding a target protein linked to the above-mentioned peptide tag. Among them, the above-mentioned two polynucleotides are preferably linked by a sequence of a polypeptide linker, wherein the above-mentioned polypeptide linker includes a site that can be cleaved by proteolytic enzymes or the like.
[0042] Furthermore, an example of the present invention provid...
Embodiment 1
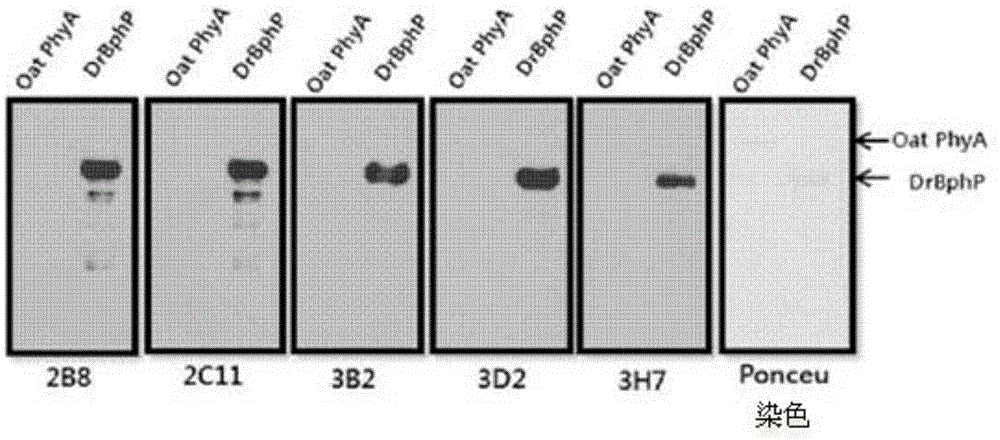
[0046] Embodiment 1: Cloning of the BphP gene and the BphN gene of Deinococcus radiodurans
[0047] By the PCR method, the gene (SEQ ID NO: 4) encoding the overall length of the bacterial phytochrome (BphP) of Deinococcus radiodurans (Deinococcus radiodurans) and the polypeptide ( BphN) gene (SEQ ID NO: 5) was cloned. Specifically, using the gene (SEQ ID NO: 4) encoding the overall length of the bacterial phytochrome protein (BphP) of Deinococcus radiodurans as a template, using primers for cloning the BphP gene or primers for cloning the BphN gene, And Ex-Taq (TAKARA) polymerase, carry out the PCR of about 30 cycles, thereby obtain the amplified BphPDNA product and BphNDNA product. Table 1 below shows the primers used to obtain the BphPDNA product and the BphNDNA product. Primers appearing in Table 1 below and all primers described later were prepared by Bioneerco. (KR).
[0048] Table 1
[0049]
[0050]The amplified PCR product was electrophoresed (running) on 1%...
Embodiment 2
[0052] Example 2: BphP protein and BphN protein of Deinococcus radiodurans (Deinococcus radiodurans) Express
[0053] After the transformed BL21 competent cells were cultured on LB medium, the grown colony (colony) was inoculated in LB medium containing kanamycin, and then inoculated at 37°C for about 8 hours Cultivate (seed culture). Inoculate the cultured cells in LB medium containing kanamycin and cultivate to OD 600 After the value reached a concentration of 0.6, IPTG (isopropyl-1-thio-β-D-galactopyranoside) was added to a final concentration of 0.5 mM, and protein expression was induced at 25° C. for 6 hours. Next, the cells were centrifuged, the supernatant was removed, and the cell pellet was resuspended with binding buffer (100 mM Tris-Cl, 150 mM NaCl, and 10 mM imidazole, pH 8.0). Then, the resuspended cell mass was sonicated to break the cells, and after centrifugation again, only the supernatant was separated, and Ni + - NTA column (QIAGEN) purified the prote...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com