Extraction method and application of ergosterol monomer compound in Armillarialuteo-virens
A technology of Armillaria japonica and ergosterol, applied in directions such as steroids, organic chemistry, drug combination, etc., can solve the problems such as restricting the research and application of ergosterol, less ergosterol content, and complicated steps, and achieves the advantages of separating The effect of operation, reagent consumption is small, and the process is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: Extraction of ergosterol monomer compound in Armillaria chrysanthemum
[0052] 1. Raw materials and materials:
[0053] The fruiting bodies of Armillaria chrysogenum were picked from Qilian, Qinghai and identified.
[0054] 2. Reagents:
[0055] Absolute ethanol was purchased from Tianjin North Tianyi Chemical Reagent Factory; chromatographic grade ethyl acetate and n-hexane were purchased from Honeywell Company.
[0056] 3. Instruments and equipment:
[0057] The ultrafine pulverizer was purchased from Shandong Sanqing Stainless Steel Equipment Co., Ltd.; the desktop refrigerated centrifuge was purchased from Thermo Company; the 30-liter Chinese medicine extraction tank was purchased from Shanghai Shunyi Experimental Equipment Co., Ltd.; the constant temperature blast drying oven was purchased from Shanghai Yiheng Scientific Instruments Co., Ltd.; rotary evaporator Shanghai Yarong Biochemical Instrument Factory; silica normal-phase silica gel chromatog...
Embodiment 2
[0065] Embodiment 2: Confirmation of ergosterol monomer compound
[0066] 1. Purity analysis by HPLC:
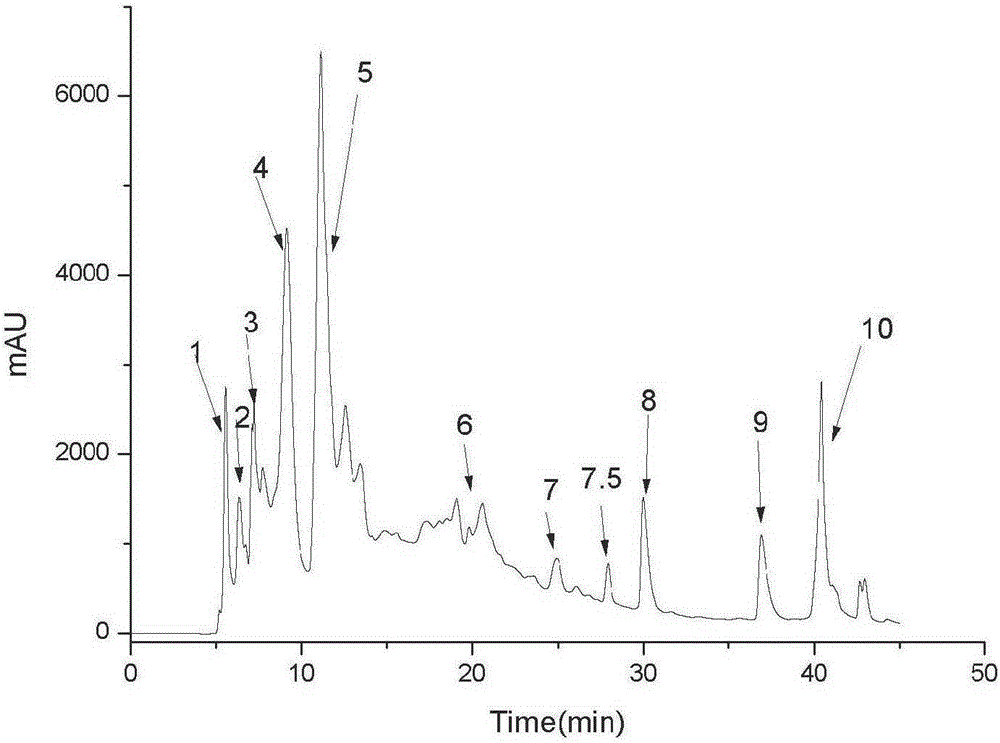
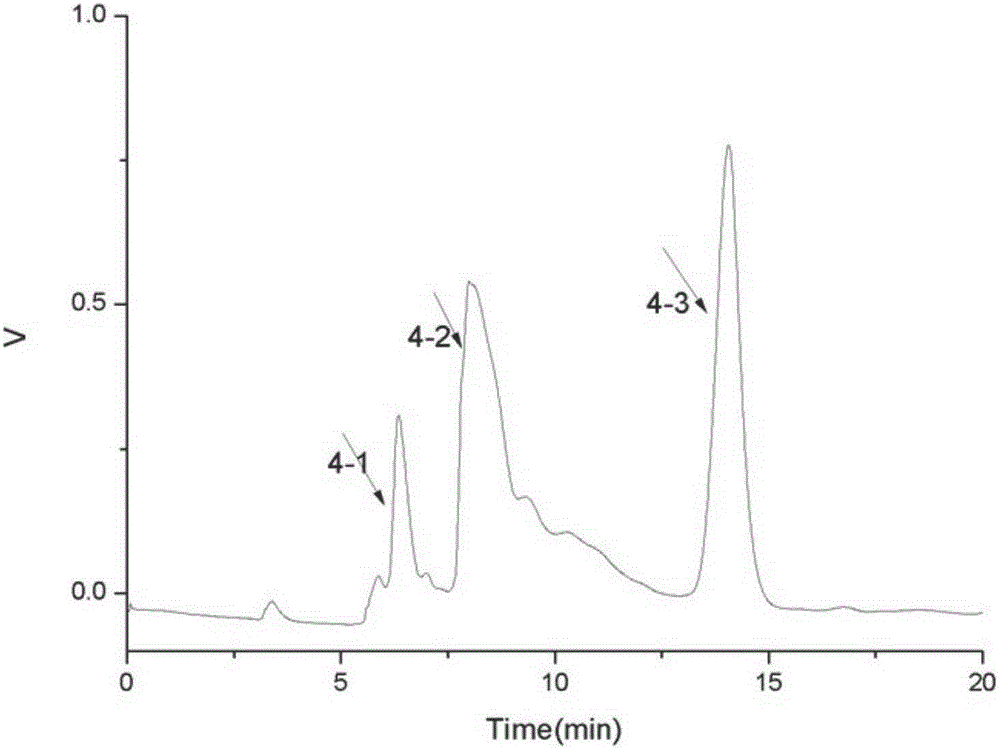
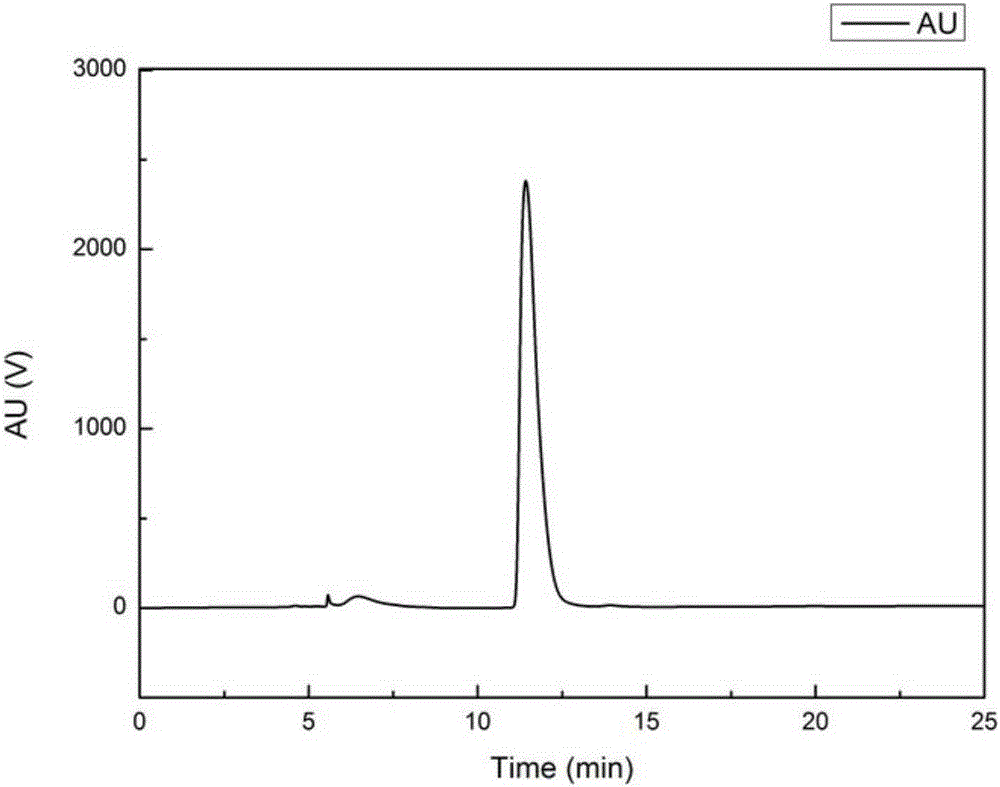
[0067] The object component that embodiment 1 obtains ( figure 2 No. 4-3 peaks) were analyzed by HPLC for purity. After HPLC detection, the purity reaches 95.23%. It is a single compound, and the HPLC spectrum is shown in image 3 .
[0068] 2. Mass spectrometry analysis:
[0069] For the purpose component ( figure 2 4-3 peak) for mass spectrometry analysis: Hanmeng Biotechnology (Tianjin) Co., Ltd., mass spectrogram see Figure 4 . EI-MSm / z: 271(M+H) + , 269 (M-H) + .
[0070] 3. NMR analysis:
[0071] For the purpose component ( figure 2 No. 4-3 peak) in NMR analysis: by carbon spectrum, hydrogen spectrum, COZY correlation spectrum (such as Figure 5-8 ) evidence analysis obtained the following results:
[0072] 1D 1 In the HNMR spectrum (CDCl 3 ,400MHz) data results are as follows: δ: 5.66 (1H, d, J = 5.5Hz, H-6), 5.41 (1H, d, J = 5.5Hz, H-7), 5.22 (2H, ...
Embodiment 3
[0076] Embodiment 3: Extraction of ergosterol monomer compound in Armillaria chrysanthemum
[0077] The raw materials, materials, reagents, instruments and equipment used in this embodiment are the same as those in Embodiment 1.
[0078] The extraction of the ergosterol monomer compound in Armillaria chrysanthemum in this embodiment comprises the following steps:
[0079] (1) Armillaria chrysanthemum fruiting body is used as raw material, dried in the shade at room temperature, dried and dehydrated at 70°C, and then ultrafinely pulverized at -20°C for 1 min to obtain Armillaria chrysanthemum ultrafine powder.
[0080] (2) Take 3kg of the above-mentioned Armillaria chrysanthemum superfine powder, soak it in ethyl acetate three times, soak for the first time: add 21L ethyl acetate to 3kg Armillaria chrysanthemum superfine powder, soak for 48h at 25°C, after soaking, 20 Centrifuge at 4,000 rpm for 30 min at ℃, collect the supernatant and residue respectively, concentrate the sup...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com