Halohydrin dehalogenase and its use in synthesis of statin drug intermediate
A halohydrin dehalogenase, halohydrin technology, applied in the field of bioengineering, can solve the problems of inappropriate reaction conditions, high enzyme dosage, long reaction steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1: the enzyme activity assay of dehalogenase
[0073] Activity of halohydrin dehalogenase: The catalytic efficiency of the enzyme is evaluated by measuring the concentration of halide ions in a reaction system containing 50mM Tris-SO4Buffer and a certain concentration of substrate.
[0074] Reagent I: 0.25MNH 4 Fe(SO 4 ) 2 Soluble in 9MHNO 3 ; Dilute with 8 times the volume of triple distilled water.
[0075] Reagent II: a saturated solution of Hg(SCN) dissolved in absolute ethanol.
[0076] (1) The substrate is 2-bromoethanol, and potassium bromide or sodium bromide is used as a standard curve; its absorbance at 460 nm is measured with an ultraviolet spectrophotometer, and water is used as a blank control.
[0077] (2) Another approach is to use chlorinated substances as substrates, and use potassium chloride or sodium chloride as a standard curve;
Embodiment 2
[0078] Embodiment 2: screening dehalogenase
[0079] Soil sample DNA (ChromaSpinTE-1000, ClontechLaboratories, Inc., USA) was collected, partially digested with Sau3AI, and 2-8kb fragments were collected by electrophoresis, recovered and ligated into the BamHI site of pUC19 to obtain a plasmid library. Transform the library into E.coliDH10b and smear it on an LB plate containing 100ug / mL ampicillin, select positive clones and transfer them to a 96 deep-well plate with 500uLLB (100ug / mL ampicillin), culture at 37°C for 4 hours, then add 1mMIPTG Induction, continue culturing overnight at 30°C; then take 50uL of each deep-well culture into a new 96-well plate containing 50mM sodium phosphate buffer (pH7.5), freeze and thaw repeatedly at -80°C to lyse the bacteria; add 2mM 6-chloro- tert-butyl 3,5-dicarbonylhexanoate and reagent I and reagent II in Example 1 were incubated at 30°C for 4 hours, and the absorbance was measured at 460nm, and the deep-well culture corresponding to the...
Embodiment 3
[0080] Embodiment 3: Construction and expression of dehalogenase recombinant bacteria
[0081]A primer pair P1 (nucleotide sequence: SeqID NO: 2) and P2 (nucleotide sequence: SeqID NO: 3) was synthesized. P1 and P2 were used to clone the full-length halohydrin dehalogenase gene.
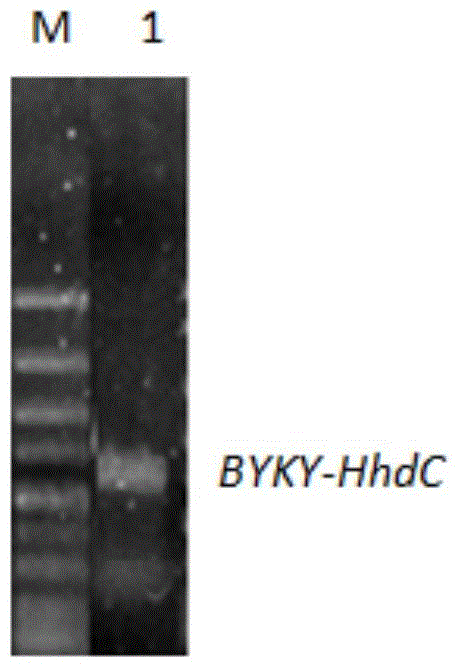
[0082] The PCR system is as follows: 10×KOD-PlusPCR buffer 2μL, 25mMMgSO4 1.2μL, 2mMdNTP 2μL, KOD-PlusPCR high-fidelity enzyme 0.3μL, DNA template 0.5μL (including DNA template 0.1μg), ddH2O 13μL, P1 and P2 each 0.5μL (10mmol / L) . PCR amplification steps are: (1) 95°C, pre-denaturation for 3min; (2) 98°C, denaturation for 15s; (3) 58°C annealing for 30s; (4) 72°C extension for 1min; steps (2) to (4) repeated 30 times; (5) Continue extending at 72°C for 10 minutes, then cool to 4°C. The PCR product was purified by agarose gel electrophoresis, and the target band in the 700-800bp range was recovered using an agarose gel DNA recovery kit (see figure 1 ), obtained a complete gene sequence, which was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com