Biflavone compound and application thereof to preparation of medicine for treating cancer
A compound and cancer technology, applied in the field of cancer treatment and drug preparation, can solve the problems of unclear material basis, lack of preparation of high-content total flavonoid active compounds, uncontrollable quality, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 Preparation of Compound 1
[0063] 1. Sample processing of Shishangbai
[0064] After cutting the dry whole plant of Shishangbai into small segments less than 5mm, it is extracted with 70% ethanol by condensing and refluxing, and concentrated by rotary evaporation at low temperature (40-50°C) to obtain ethanol extract extract. Add 10 times the volume (m / v) of double-distilled water to suspend the extract, and extract it with equal volumes of petroleum ether, dichloromethane and ethyl acetate in sequence. paste. Take the ethyl acetate extract and perform the following steps to prepare the compound of the present invention.
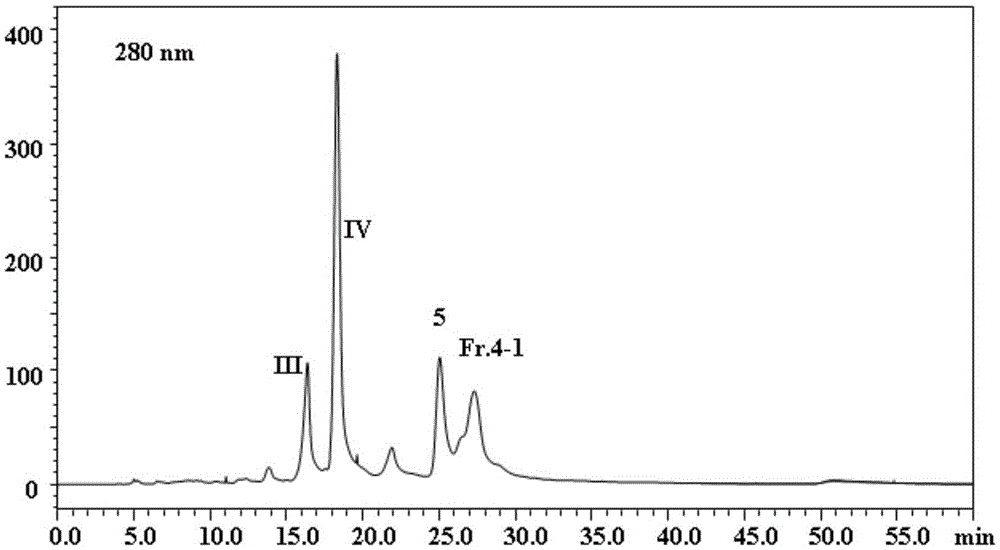
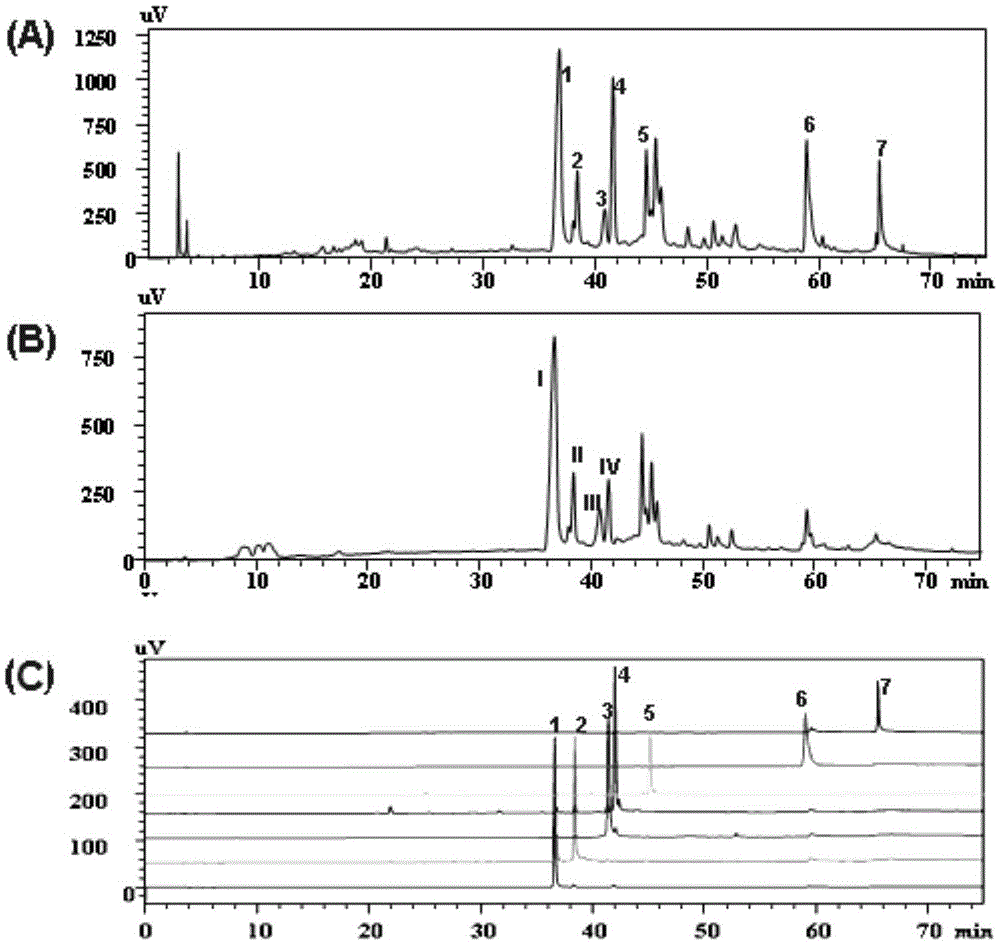
[0065] 2. HPLC preparation and separation
[0066] The preparative column is an Agilent SB-C18 column (250mm×21.2mm, 7μm), mobile phase acetonitrile-water (44:56, v / v), flow rate 7ml / min, detection wavelength 280nm, room temperature, sample volume is 100μL. Separated by high performance preparative liquid chromatography, such as figure 1 ( fig...
Embodiment 2
[0082] Example 2 Evaluation of in vitro anticancer activity of compound 5
[0083] The compound 5 prepared above was used to evaluate the anticancer activity in the following human tumor cell lines in vitro.
[0084] 1. Compound solution preparation: accurately weigh a certain amount of compound 5, dissolve it with dimethyl sulfoxide (DMSO), and dilute to the concentration of 500, 250, 125, 62.5, 31.25 μg / mL with the growth medium of each cell. 5 concentrations of the test solution, the positive control drug doxorubicin preparation solution was sequentially diluted with the growth medium of each cell into 3 concentration test solutions of 5, 2.5, 1.25μM.
[0085] 2. Cell lines and cell culture
[0086] Seven human tumor cell lines, including 5 adherent tumor cell lines: human gastric cancer cell line (MKN-45), non-small cell lung cancer (A549), nasopharyngeal carcinoma (CNE1, CNE2), large cell lung cancer (PC -9); 2 kinds of tumor cells are suspension type: human acute promyelocytic ...
Embodiment 3
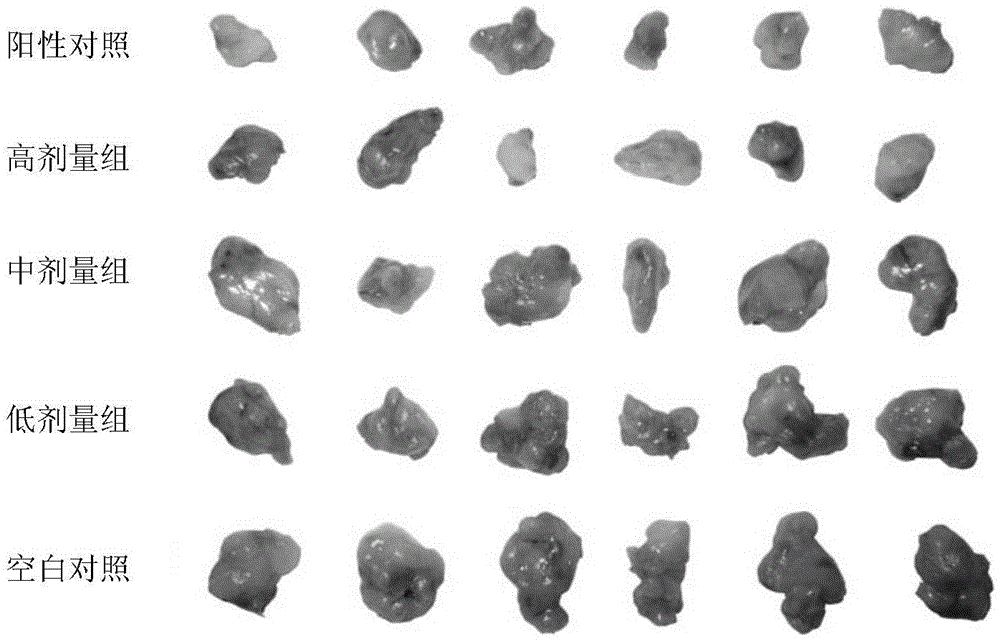
[0096] Example 3 Evaluation of anti-cancer activity of compound 5 in animal model
[0097] The compound 5 prepared above was used to evaluate the anticancer activity in the following human tumor cell lines in vitro.
[0098] 1. Cell lines and laboratory animals
[0099] Human non-small cell lung cancer cell line A549 cells were purchased from the cell bank of the Shanghai Chinese Academy of Sciences and cryopreserved by the School of Pharmacy, Fujian Medical University.
[0100] BALB / c nude mice aged 3-4 weeks, weighing 18±2g (male), purchased from Shanghai Slack Laboratory Animal Co., Ltd., laboratory animal license number: SCXK (Shanghai) 2012-0002, animal certificate number: 2007000537438 .
[0101] Nude mice are housed in a SPF-level laminar flow system every 6 cages. Nude mice are given standard food and drinking water every day, keep light and dark cycle experiment (12 / 12h), room temperature 25±2℃, humidity 50±10 %, change the drinking water and litter after autoclaving every da...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com