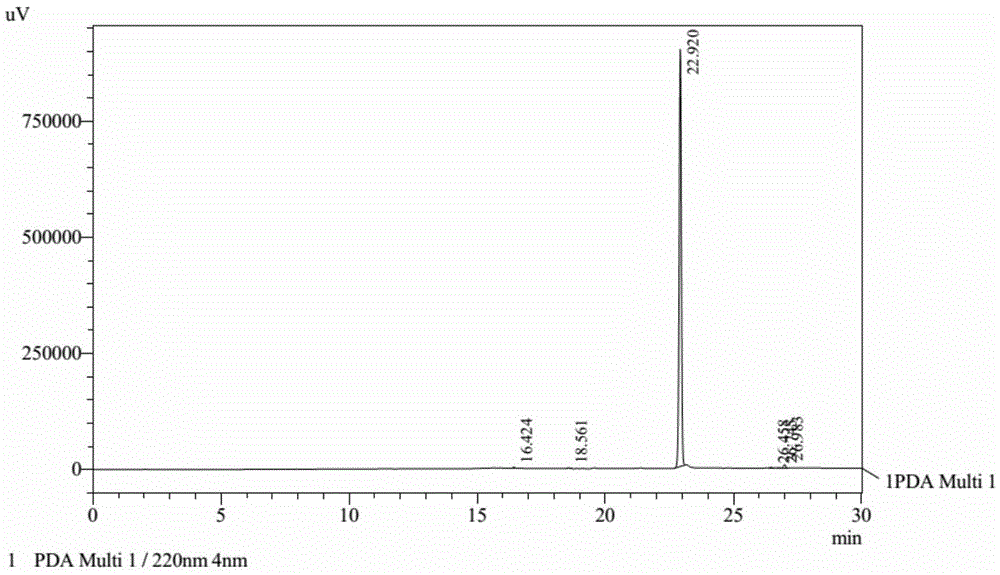
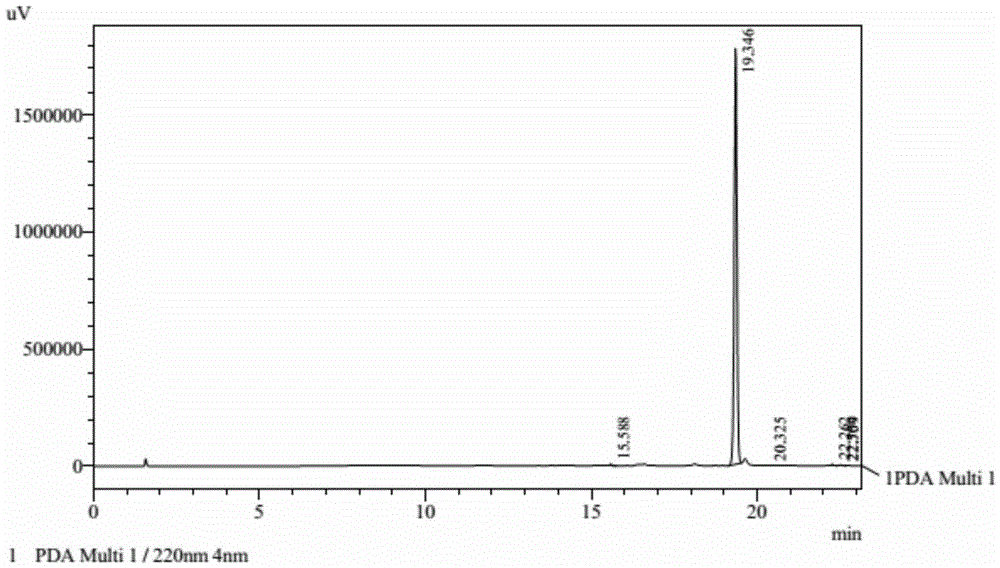
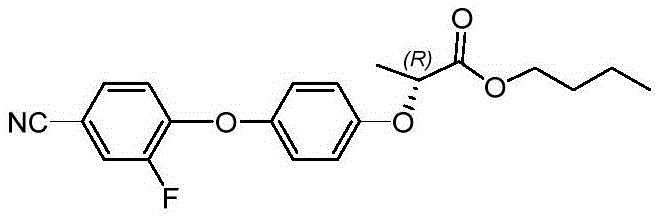
Method for preparing cyhalofop-butyl
A technology of cyhalofop-ethyl and propionic acid, which is applied in the field of preparation of cyhalofop-methyl, can solve the problems of unrecoverable by-products of p-toluenesulfonic acid, high requirements for experimental equipment and operation, and increased safety hazards, etc., to reduce the discharge of three wastes Quantity, avoiding recrystallization and impurity removal operations, and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation method of embodiment 1 cyhalofop-ethyl
[0045] 1) etherification reaction
[0046] Put 100mL of N,N-dimethylacetamide and 60g (0.42mol) of potassium carbonate into a 250ml four-necked flask, and then add 26g (0.14mol) of (R)-2-(4-hydroxyphenoxy)propionic acid in batches ), after the feeding is completed, 20 g (0.14 mol) of 3,4-difluorobenzonitrile is added, then the temperature is raised to 120° C., and the reaction is kept for 5 hours, and the reaction ends. Distill under reduced pressure to remove the solvent, drop to room temperature and add 150 mL of water to dissolve, adjust the pH value to 4-5 with 30% dilute sulfuric acid, stir to precipitate a solid, and filter to obtain (R)-2-[4-(2-fluoro-4-carbonitrile base)-phenoxy]-propionic acid for subsequent use.
[0047] 2) Esterification reaction
[0048] Drop into above-mentioned intermediate (R)-2-[4-(2-fluoro-4-nitrile group)-phenoxy group]-propionic acid, 200mL benzene, n-butanol 18g (0.24mol) an...
Embodiment 2
[0052] The preparation method of embodiment 2 cyhalofop-ethyl
[0053] 1) etherification reaction
[0054] Add 100mL of N,N-dimethylacetamide and 80g (0.58mol) of potassium carbonate into a 250ml four-necked flask, and then add 26g (0.14mol) of (R)-2-(4-hydroxyphenoxy)propionic acid in batches. ), after the feeding was completed, 25 g (0.18 mol) of 3,4-difluorobenzonitrile was added, then the temperature was raised to 100° C., and the reaction was kept for 6 hours, and the reaction ended. Remove the solvent by distillation under reduced pressure, add 200 mL of water to dissolve at room temperature, adjust the pH value to 4-5 with 20% hydrochloric acid, stir to precipitate a solid, and filter to obtain (R)-2-[4-(2-fluoro-4-nitrile )-phenoxy]-propionic acid for subsequent use.
[0055] 2) Esterification reaction
[0056] Drop into above-mentioned intermediate (R)-2-[4-(2-fluoro-4-nitrile group)-phenoxy group]-propionic acid, 200mL toluene, n-butanol 15g (0.20mol) and Concent...
Embodiment 3
[0057] Embodiment 3 A kind of preparation method of cyhalofop-methyl, comprising:
[0058] 1) etherification reaction
[0059] Put 100mL of N,N-dimethylformamide and 40g (0.29mol) of potassium carbonate into a 250ml four-necked flask, and put 26g (0.14mol) of (R)-2-(4-hydroxyphenoxy)propionic acid in batches , after the feeding is completed, 20 g (0.14 mol) of 3,4-difluorobenzonitrile is added, then the temperature is raised to 105° C., and the reaction is kept for 6 hours, and the reaction is completed. Remove the solvent by distillation under reduced pressure, add 150 mL of water to dissolve at room temperature, adjust the pH value to 4-5 with 30% dilute sulfuric acid, stir to precipitate a solid, and filter to obtain the intermediate (R)-2-[4-(2-fluoro-4 -Nitryl)-phenoxy]-propionic acid for later use.
[0060] 2) Esterification reaction
[0061] Put the above-mentioned intermediate (R)-2-[4-(2-fluoro-4-nitrile)-phenoxy]-propionic acid, 200mL butanone, and 18g (0.24mol) o...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap