Preparation method of alpha, beta-unsaturated carboxylic acid compounds
A technology of compounds and carboxylic acids, applied in the field of α, can solve problems such as very good, unspecified functional group compatibility effect, narrow substrate range, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The present invention provides a kind of α, the preparation method of β-unsaturated carboxylic acid compound, comprising: under the condition that nickel-containing catalyst, phosphine ligand, acid anhydride and organic solvent exist, compound shown in formula (I) and Formic acid reacts to obtain the α shown in formula (II), the β-unsaturated carboxylic acid compound;
[0036]
[0037] where the R 1 with R 2 each independently selected from one of hydrogen, alkyl, substituted alkyl, alkenyl, substituted alkenyl, aryl and substituted aryl.
[0038] The reaction formula for this reaction is as follows:
[0039]
[0040] The present invention has no special limitation on the sources of all raw materials, which can be commercially available.
[0041] Wherein, the nickel-containing catalyst is a nickel-containing catalyst well known to those skilled in the art, and there is no special limitation. In the present invention, nickel chloride, bis(1,5-cyclooctadiene) nic...
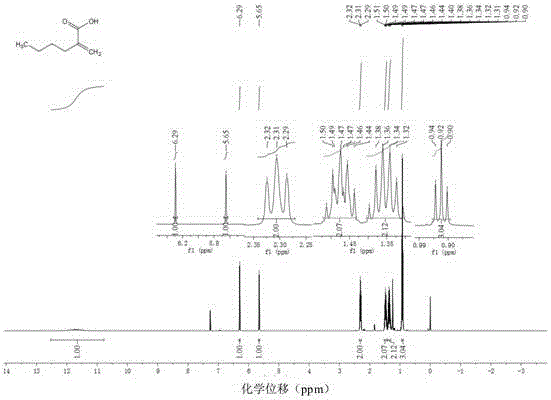
Embodiment 1
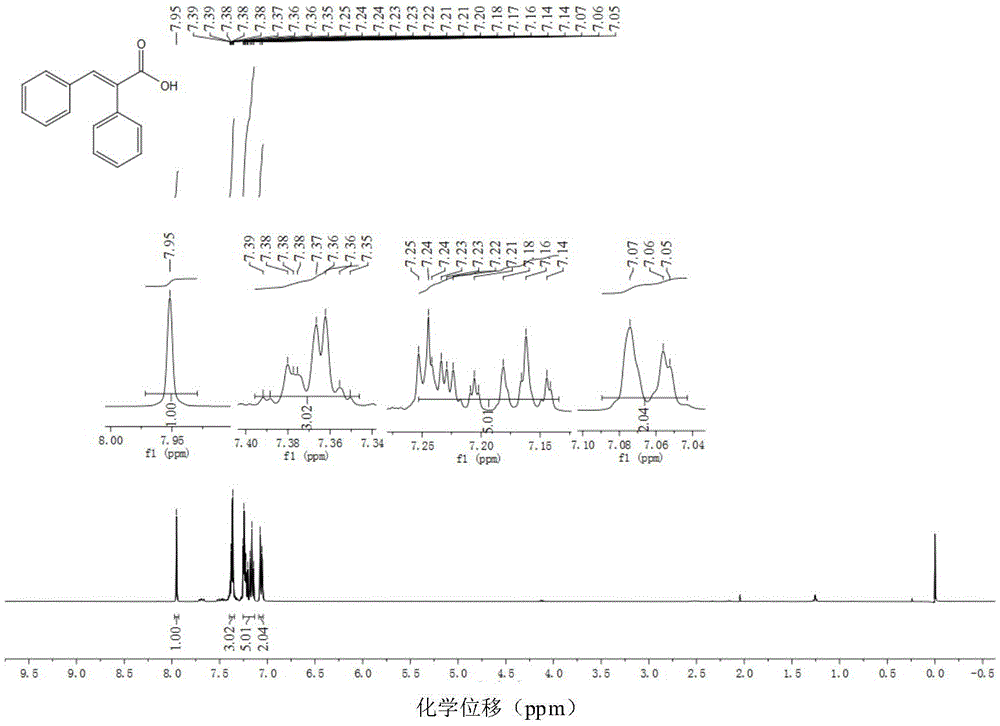
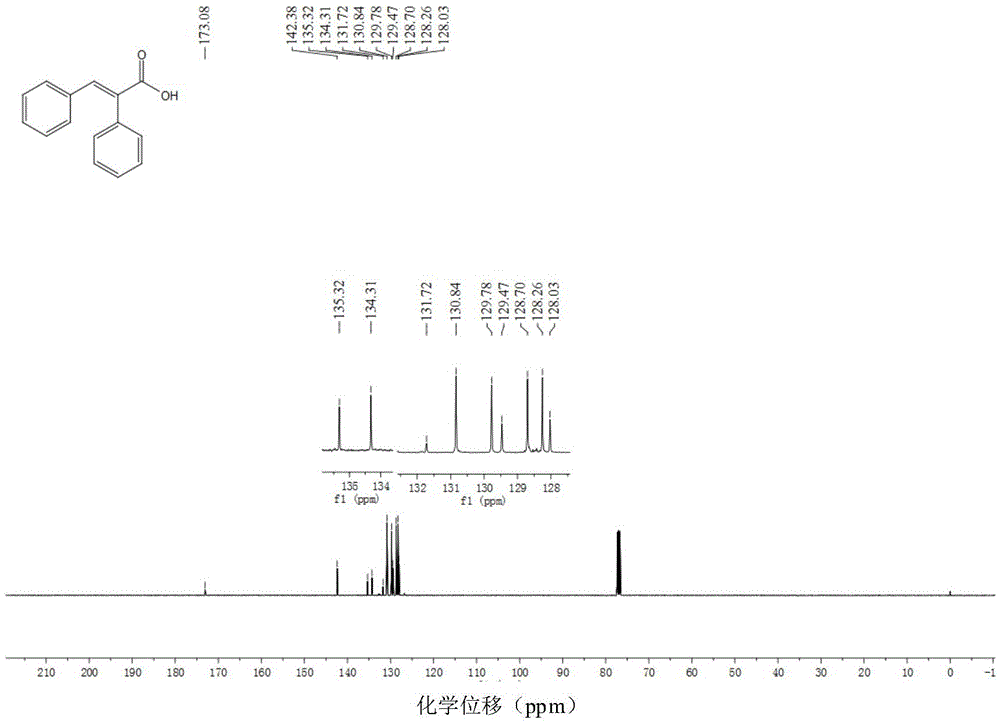
[0054] Example 1: Preparation of (E)-2,3-diphenyl α, β-unsaturated carboxylic acid
[0055] Reaction formula:
[0056]
[0057] Add di(acetylacetonate)nickel (5mol%, 6.5mg) and 1,2-bis( Diphenylphosphino)benzene (7mol%, 15.6mg), completely replace the air in the tube with argon three times, then add 1ml of toluene, toluene (0.50mmol, 89.2mg), formic acid (1.1mmol, 50.6mg) and pivalic anhydride (20mol%, 0.1mmol, 18.4mg); The reaction system was heated to 100°C under an oil bath and stirred continuously for 24 hours (use IKA magnetic stirrer, RCT basic type, stirring speed 500 rpm / minute). After the reaction was completed, the system was cooled to room temperature. The reaction solution was diluted with ethyl acetate, and then the diluted reaction solution was concentrated by rotary evaporation (BUCHI Co., Ltd., BUCHI rotary evaporator R-3). The concentrated residue was chromatographically separated by a chromatographic column (Beijing Xinweier Glass Instrument Co., Ltd....
Embodiment 2
[0060] Example 2: Preparation of (E)-2,3-di-p-methylphenylacrylic acid
[0061] Reaction formula:
[0062]
[0063] The method is the same as Example 1, the yield is 86%, and the purity is >99%.
[0064] The (E)-2,3-di-p-methylphenylacrylic acid obtained in Example 2 is analyzed by nuclear magnetic resonance, and the results are obtained: 1 HNMR (400MHz, CDCl 3 )δ7.89(s,1H),7.19(d,J=7.8Hz,2H),7.13(d,J=7.9Hz,2H),6.99(s,4H),2.39(s,3H),2.28( s,3H); 13 CNMR (101MHz, CDCl 3 ) δ 172.66, 142.23, 139.83, 137.73, 132.50, 131.65, 130.88, 130.46, 129.59, 129.51, 129.03, 21.38.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com