Anti-rheumatism traditional Chinese medicine quality evaluation method based on bioavailability
A technology for biological potency and quality evaluation, applied in biochemical equipment and methods, microbial determination/inspection, biological testing, etc. The method has not been reported and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Determination of related biological activities of Qufeng Zhitong Tablets, Tripterygium wilfordii Polyglycoside Tablets and Total Radix Paeony Capsules
[0065] 1. Nitric oxide (NO) kit method to determine the biological activity of drugs in inhibiting chondrocyte NO secretion
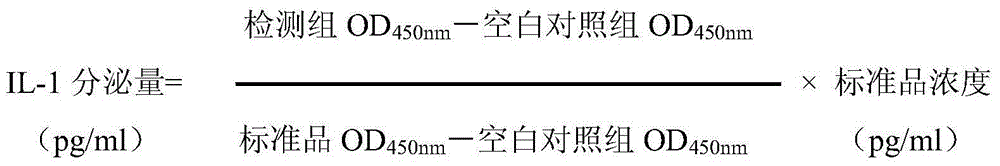
[0066] Through the chondrocyte method, based on the culture of primary chondrocytes, the degradation of the chondrocyte matrix was induced by interleukin (IL)-1β stimulation in vitro as a pharmacological model, and the nitric oxide (NO) nitrate reductase assay kit was used to detect NO content, the inhibition rate of chondrocyte NO secretion was used as an index.
[0067] Specific experimental method: Inoculate chondrocytes cultured in vitro into 96-well plates, add IL-1β at a concentration of 100ng / ml after 24 hours of culture, set 3 replicates for each concentration, set up a blank control group in parallel, and place at 37°C , 5% CO 2 Cultivate in the incubator for 24 hours and set...
Embodiment 2
[0102] Example 2 Determination of Biological Potency of Qufeng Zhitong Tablets Inhibiting COX-2 Activity
[0103] A. Reference substance solution preparation: take celecoxib as the reference substance, dissolve in the reaction system of 10 μl COX-2 (final concentration is 0.48U / μl)+100 μMAA+2.1mMTMPD, and filter;
[0104] B. Preparation of test solution: prepare Qufengzhitong tablet test solution as previously described;
[0105] C. need testing solution and reference substance solution are diluted into the dosage group of different concentration with same dose distance, dose distance is 0.5; The solvent used for dilution is 10 μ l COX-2 (final concentration is 0.48U / μ l)+100 μ MAA+2.1mMTMPD;
[0106] D. Data processing and potency calculation: The COX-2 activity inhibition rate of the different dose groups of the test product and the reference substance was respectively measured by using the microplate reader method described in Example 1, and the calculation of the test pro...
Embodiment 3
[0109] Example 3 Determination of biological potency of tripterygium glycosides tablets inhibiting chondrocyte NO secretion
[0110] A. preparation of reference substance solution: take dexamethasone as reference substance, use the DMEM culture fluid of 20% fetal bovine serum as solvent, be made into the reference substance solution of corresponding concentration, filter, standby;
[0111] B. preparation of test solution: prepare tripterygium wilfordii polyglycoside tablets need test solution as described above;
[0112] C. need testing solution and reference substance solution are diluted into dosage groups of different concentrations with the same dosage interval, dosage interval is 0.5, dilutes with the DMEM nutrient solution of 20% fetal bovine serum;
[0113] D. Data processing and titer calculation: adopt the nitric oxide (NO) kit method described in embodiment 1 to measure respectively the chondrocyte NO secretion inhibitory rate that test article and reference substanc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com