New process for clean production of pmida and cyclic utilization of byproducts
A technology of diglyphosate and raw materials, which is applied in the field of clean production process of glyphosate intermediate diglyphosate, which can solve the problems of increased ammonia consumption, large initial investment, and biochemical difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
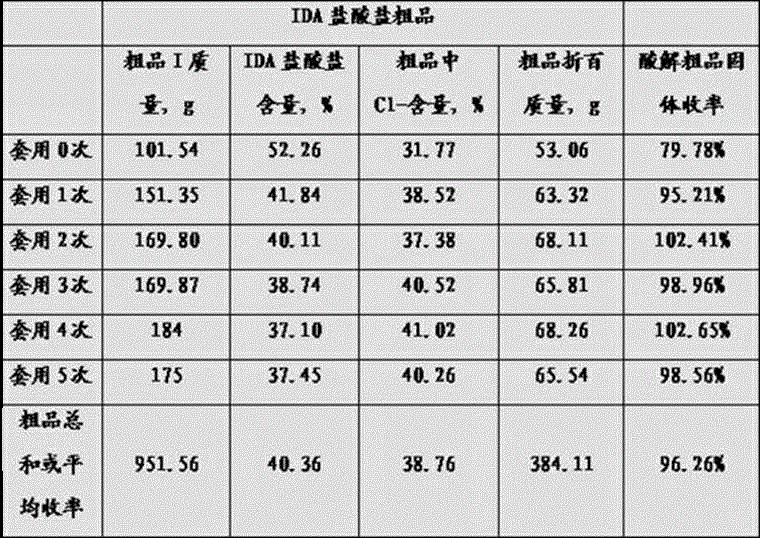
[0070] Example 1 The acid solution of iminodiacetonitrile is applied mechanically to prepare the crude product of iminodiacetic acid hydrochloride.
[0071] Add 300g of 32% hydrochloric acid and 50g of water into a 500mL four-necked flask, and preheat it to 60-80°C, add 50g of 95% iminodiacetonitrile solid or solution to the preheated hydrochloric acid at a uniform speed within 30min, and the addition is complete Insulate at 95-120°C for 30 minutes, cool down and crystallize and separate to obtain 101g of crude iminodiacetate hydrochloride, 295g of acidolysis mother liquor; add 50g of water to the acidolysis mother liquor, pass in 55g of HCl gas, and preheat to 60 -80°C, add 50g of 95% iminodiacetonitrile solid or solution to the preheated acidolysis mother liquor with HCl gas at a uniform speed within 30 minutes, after adding, keep it warm at 95-120°C for 30 minutes, cool down to crystallize and separate, Obtained 151g of iminodiacetate hydrochloride crude product, 296g of ac...
Embodiment 2
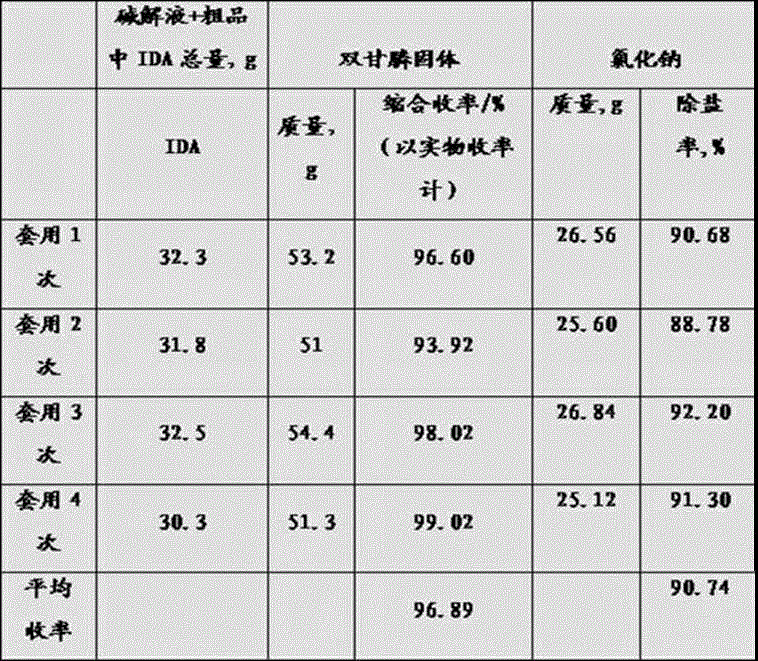
[0073] Example 2 Alkaline hydrolysis of iminodiacetonitrile and addition of iminodiacetochloride hydrochloride crude product condensation embodiment
[0074] Mix 44g of 96% sodium hydroxide and 150g of water into a 500mL four-neck flask, stir and maintain the temperature at 50-80°C, add 50g of 95% iminodiacetonitrile solid or solution into the sodium hydroxide solution at a uniform speed, and add The time is 0.5-1h. After the feeding is completed, keep the temperature at 50-80°C for 30min, and gradually raise the temperature of the alkaline solution to 100-110°C for deamination for about 1h. Add 38g (IDA%=40.36%, acidity is 50% based on HCl) mixed iminodiacetic acid hydrochloride crude product in Example 1, partially acidify the alkaline solution and continue heating for deamination until the steam pH=7 Sampling after -7.5 to track the determination of free ammonia and free NH 3 -N%=0.03%, after deamination, add 60g of hydrochloric acid (37%) to continue to acidify the system...
Embodiment 3
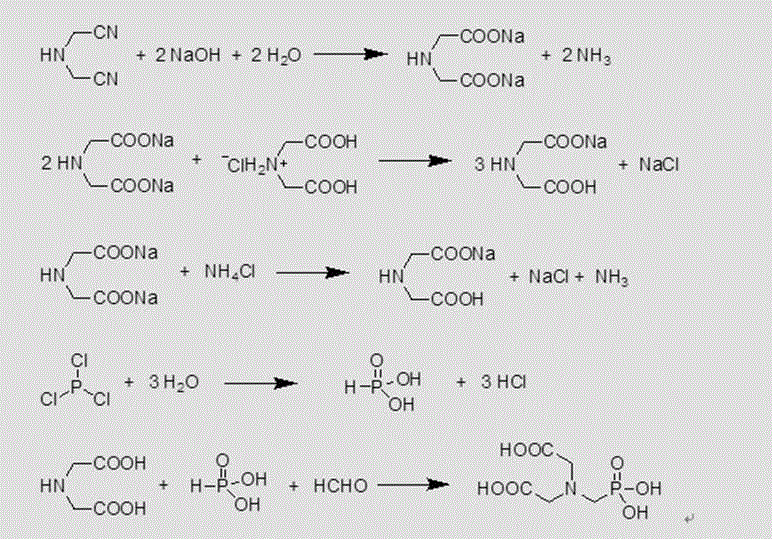
[0075] Example 3 Condensation of crude product of iminodiacetonitrile disodium salt by adding iminodiacetate hydrochloride
[0076] Take 200g of the disodium salt feed solution after the alkaline hydrolysis of iminodiacetonitrile in the workshop, in which the iminodiacetic acid content is 25.09%; take 17.30g of the crude diacid product, in which the IDA content is 48.38%, and mix the two into 500mL four-port In the flask, gradually increase the temperature to 100-110°C for deamination, after deamination to steam pH=7-7.5, take samples to track and measure free ammonia and free NH 3 -N%=0.01%, after the deamination is completed, replenish water until the concentration of iminodiacetic acid is 27.5%, add hydrogen peroxide and activated carbon, and keep warm at 80-100°C for 30 minutes for decolorization, and the decolorized feed liquid is between 60-70°C Keep warm and slowly add 72.30g of 98.26% phosphorus trichloride solution dropwise. After the dropwise addition, keep warm for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com