Recombinant protein A and applications thereof
A technology of recombinant protein and staphylococcal protein, which is applied in the field of new recombinant Staphylococcus aureus protein A and its application in protein separation and purification, can solve the problems of unsatisfactory and unstable, and achieve improved stability and high alkali resistance performance, increase the maximum load effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Embodiment 1: Preparation of recombinant protein A polymer
[0108] a. Synthesis of recombinant protein A polymer gene
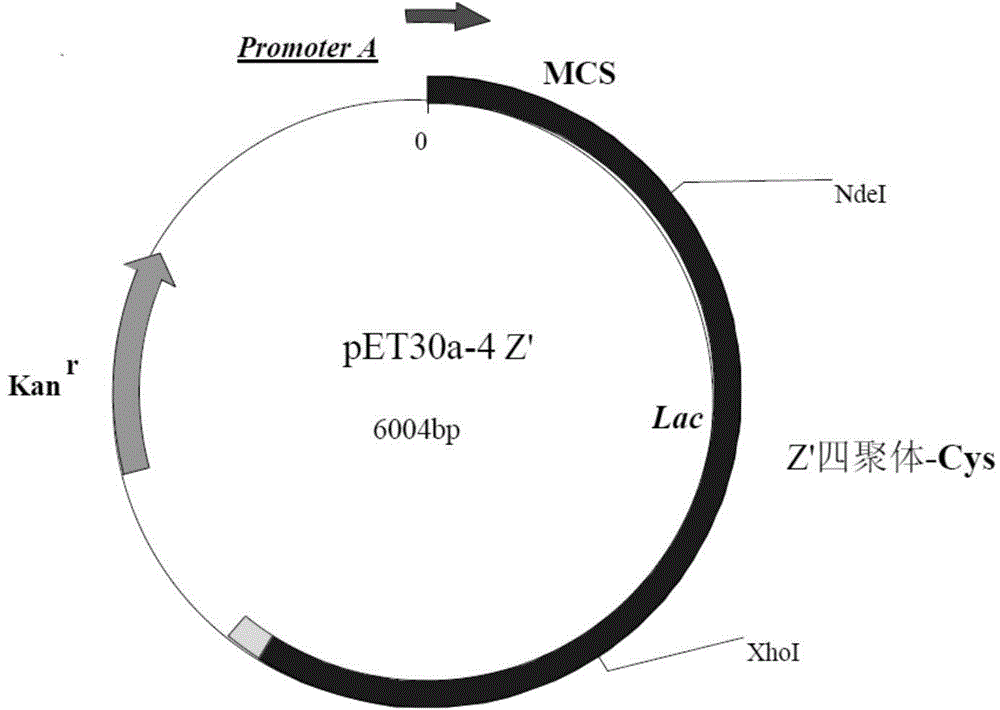
[0109] The cDNA sequence encoding the recombinant protein A polymer (as shown in SEQIDNo.1) was synthesized using the whole gene. The full length of the sequence is 768bp (entrusted to Shanghai Jierui Bioengineering Co., Ltd.), including the start codon ATG and the stop codon TAA, The synthetic sequence was ligated into the expression vector pET30a(+) to obtain an expression plasmid named pET30a-4Z', such as figure 1 shown.
[0110] b. Sequence identification and expression
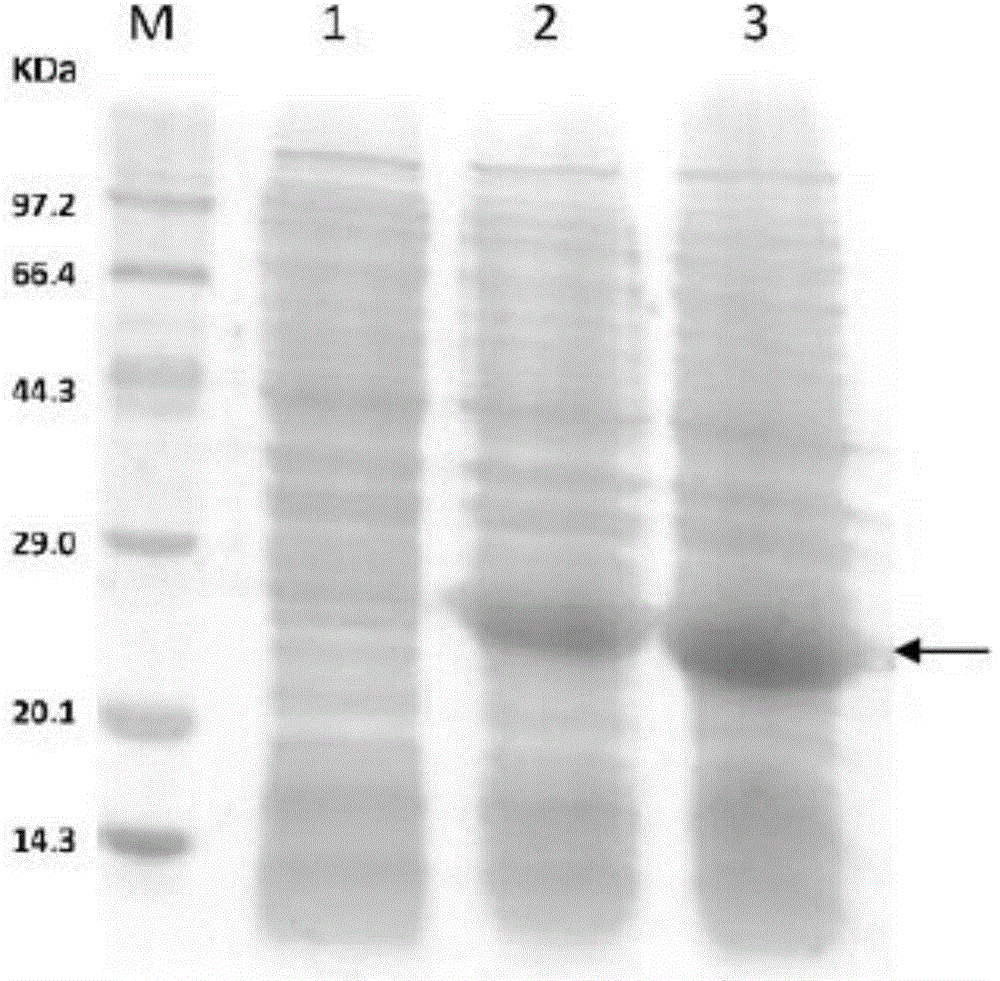
[0111] with CaCl 2 The plasmid pET30a-4Z' was transformed into Escherichia coli BL21 (DE3), grown overnight on LB plates containing 50 μg / mL kanamycin (kana for short), picked single-clonal colonies, and inserted into LB liquid medium ( 50 μg / mL kana) at 37°C, shaking at 200 rpm for 8 hours. Take 2 mL of bacterial liquid and add it to 100 mL of LB medium (50 μg / mL kana), cul...
Embodiment 2
[0122] Embodiment 2: load determination
[0123] The recombinant protein A polymer prepared in Example 1 was used as a ligand, and the monodisperse porous copolymer polystyrene-divinylbenzene (PS / DVB) microspheres were used as a carrier. In the PB buffer solution with a pH of 6-8, stirred and reacted at 25°C for 8 hours, the protein and the microsphere carrier were cross-linked through thioether bonds, and the obtained medium was washed several times with PB buffer solution to remove unbound protein, and dried in vacuum to obtain the And chromatography medium (named recombinant protein A polymer medium).
[0124] Use the cell supernatant sample (2.0g / L) expressing human IgG to carry out load determination on the following affinity chromatography medium:
[0125] Recombinant protein A polymer medium;
[0126] MabCapture TM Medium A (purchased from AB Applied Biosystems, Cat. No. 4374730) was used as a contrast medium.
[0127] The loading buffer is 10mMPB+0.2MNaCl, pH7.5. ...
Embodiment 5
[0135] Embodiment 5: Recombinant protein A polymer and recombinant wild-type protein A (rPA) alkali resistance determination
[0136] Add 10mL of 1M NaOH solution to 10mL of the recombinant protein A polymer prepared in Example 1 (6mg / mL) and mix well (the concentration of NaOH in the mixed solution is 0.5M), and then incubate in a 37°C incubator for 4hr, 8hr, 16hr, 24hr sampling, 12% SDS-PAGE electrophoresis analysis of untreated (0hr) and lye-treated samples, the sample load of each time point is 5 μg, the electrophoresis results are as follows Figure 4 In the figure (a) shown.
[0137] Take 0.6mL of recombinant wild-type protein A (Wild-type Staphylococcus aureus protein A recombinantly expressed by E.coli, the concentration is 1.2mg / mL) solution, add 5.4mL10mMPB, pH7.2, mix well, reserve 1mL of the sample, and add Add an equal volume of 1M NaOH solution (5mL) and mix evenly (the concentration of NaOH in the mixed solution is 0.5M), incubate in a 37°C incubator, take samp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com