A kind of manganese carbonate ore leaching method based on two-stage leaching
A manganese carbonate ore, two-stage leaching technology, applied in the field of hydrometallurgy of manganese carbonate ore, to achieve the effect of increasing the leaching rate, prolonging the service life of equipment, and reducing the dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
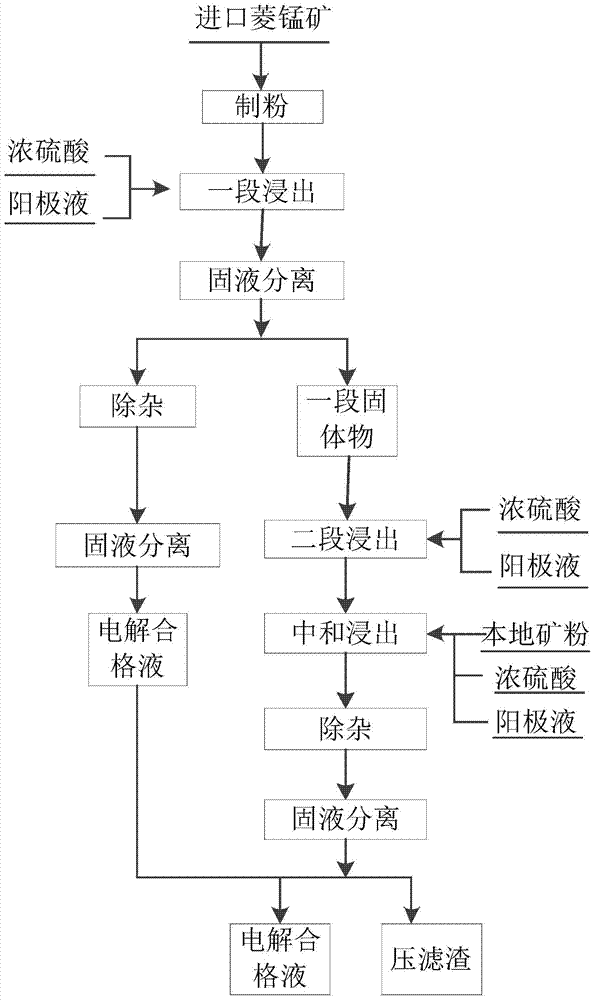
[0046] Kind of like figure 1 The leaching method of manganese carbonate ore based on two-stage leaching shown includes the following steps:
[0047] (1) After grinding 28t of high-grade manganese carbonate ore (imported ore, see Table 1 below for its grade), put it into the reaction tank, add 1t of concentrated sulfuric acid and 300m of anolyte 3 (See Table 2 for anolyte composition), the leaching reaction time is 2h, the ore leaching rate is 51.3%, and the manganese content of the leaching residue is 18.9%;
[0048] (2) After the leaching is finished, the residual acid in the reaction is detected to be 1.2g / L, and the neutralizing agent ammonia is added to neutralize it according to the concentration of the residual acid in the reaction;
[0049] (3) Use a thickener for liquid-solid separation of the neutralized pulp in step (2), separate the leachate and a section of solids of 20.05t (dry basis), and send the separated leachate (see Table 3 for composition analysis) to impurity rem...
Embodiment 2
[0069] Kind of like figure 1 The leaching method of manganese carbonate ore based on two-stage leaching shown includes the following steps:
[0070] (1) After grinding 50t of high-grade manganese carbonate ore (imported ore, whose grade is shown in Table 1 above), put it into the reaction tank, add 12t of concentrated sulfuric acid and 300m of anolyte 3 (See Table 2 for anolyte composition), the leaching reaction time is 4h, the ore leaching rate is 62.5%, and the manganese content of the leaching residue is 16.4%;
[0071] (2) After the leaching, the residual acid in the reaction was detected to be 2.1g / L, and the artificial manganese carbonate slurry and ammonia water were added to neutralize the residual acid according to the concentration of the residual acid in the reaction;
[0072] (3) The neutralized slurry in step (2) is separated into liquid and solid using a combination of thickener and filter press to separate the leachate and a section of solids 32.1t (dry basis), and th...
Embodiment 3
[0084] Kind of like figure 1 The leaching method of manganese carbonate ore based on two-stage leaching shown includes the following steps:
[0085] (1) After grinding 40t of high-grade manganese carbonate ore (imported ore, whose grade is shown in Table 1 above), put it into the reaction tank, add 13t of concentrated sulfuric acid and 300m of anolyte 3 (See Table 2 for anolyte composition), the leaching reaction time is 6h, the ore leaching rate is 85.02%, and the manganese content of the leaching residue is 8.5%;
[0086] (2) After the leaching is completed, the residual acid of the reaction is detected to be 4g / L, and the artificial manganese carbonate slurry, calcium carbonate and ammonia are sequentially added to neutralize the residual acid according to the concentration of the residual acid;
[0087] (3) The neutralized slurry in step (2) is separated from the liquid and solid by filter press, and the leachate (see Table 13 for composition analysis) and a section of solid 20t ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com