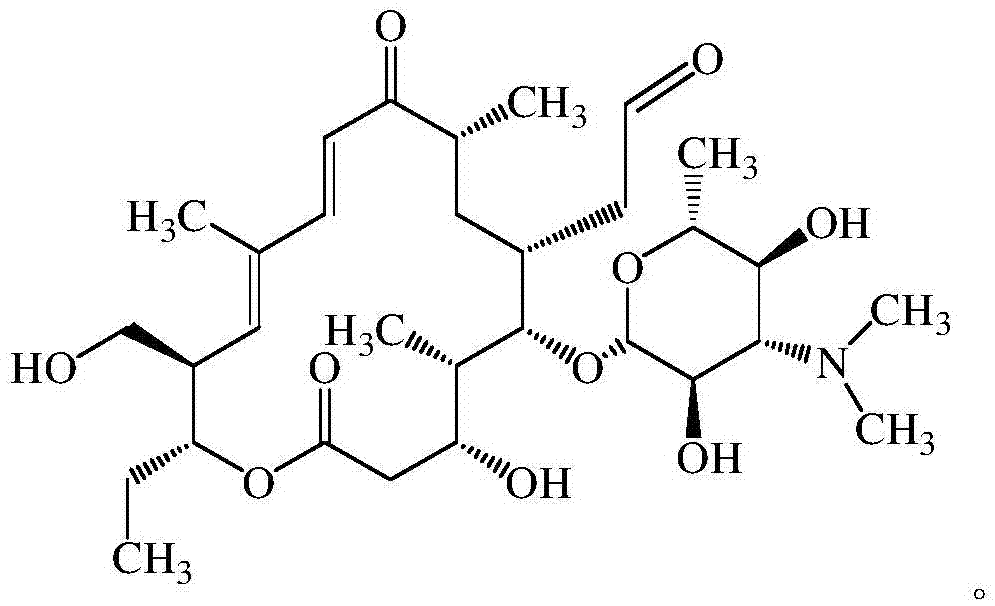
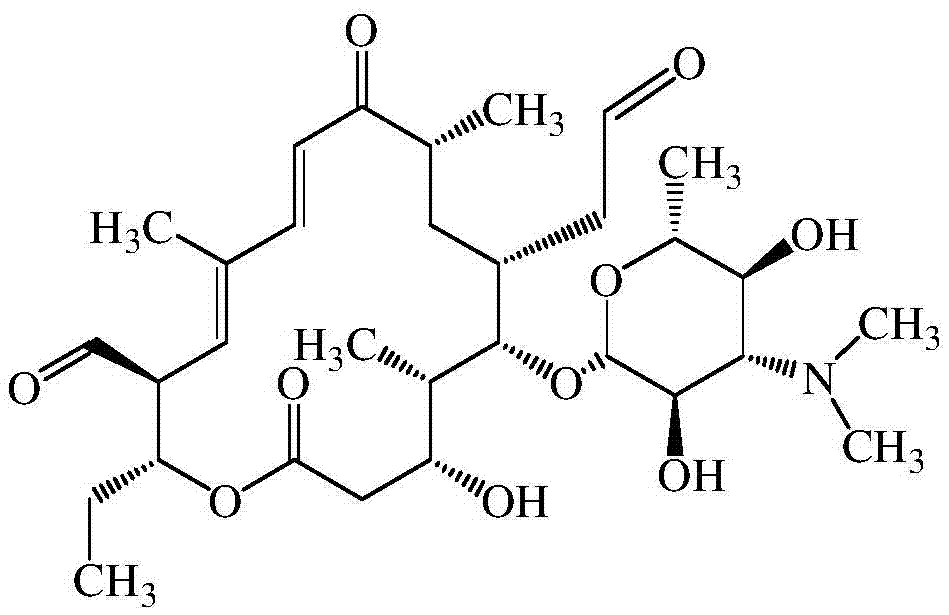
Synthetic method of tildipirosin
A synthesis method and tediloxine technology, applied in the field of biomedicine, can solve the problems of difficult to control the impurity content of the final product, complicated purification process, numerous process steps, etc., and achieve high industrial application value, high reaction yield, raw material Safe and easy to get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In the new production line of 100kg class Tadiro, the 2m 3 Put 100kg of tylosin tartrate into the glass-lined main reaction kettle, add 500kg of purified water, stir and raise the temperature to 55°C until the tylosin is completely dissolved. The pH was adjusted to 1.0 by using 24% hydrobromic acid prepared in advance, and the reaction was incubated for 8 hours.
[0030] After the reaction is completed, to 2m 3 Add 250kg of dichloromethane into the glass-lined main reaction kettle, stir evenly, then use 20% sodium hydroxide solution to adjust the pH of the water phase to 9.2, continue stirring until uniform, let stand to separate the liquid, and separate the water phase.
[0031] Add 20kg of KBr into a 500L secondary reaction kettle, then add 10kg of NaClO, add 100kg of purified water, and stir until completely dissolved. with saturated NaHCO 3 Adjust the pH to 9.0.
[0032] to 2m 3 In the main reaction kettle, add 19.55kg (1.15eq) 2,2,6,6-tetramethylpiperidine nit...
Embodiment 2
[0035] In the new production line of 100kg class Tadiro, the 2m 3 Put 91.6kg of tylosin tartrate into the glass-lined main reaction kettle, add 367kg of purified water, stir and raise the temperature to 54°C until the tylosin is completely dissolved. The pH was adjusted to 0.8 by using 26% hydrobromic acid prepared in advance, and the reaction was incubated for 10 hours.
[0036] After the reaction is completed, to 2m 3 Add 113 kg of dichloromethane into the glass-lined main reaction kettle, stir evenly, then adjust the pH of the water phase to 9.4 with 22% sodium hydroxide solution, continue stirring until uniform, let stand to separate the liquid, and remove the water phase.
[0037] Add 20kg of KBr into a 500L secondary reaction kettle, then add 11kg of NaClO, add 120kg of purified water, and stir until completely dissolved. with saturated NaHCO 3 Adjust the pH to 8.8.
[0038] to 2m 3 In the main reaction kettle, add 18.52kg (1.17eq) 2,2,6,6-tetramethylpiperidine nitr...
Embodiment 3
[0041] In the new production line of 500kg class Tadiro, the distance is 10m 3 Put 458kg of tylosin tartrate into the glass-lined main reaction kettle, add 2060kg of purified water, stir and raise the temperature to 56°C until the tylosin is completely dissolved. The pH was adjusted to 0.6 by using 25% hydrobromic acid prepared in advance, and the reaction was incubated for 9 hours.
[0042] After the reaction is completed, to 10m 3 Add 515 kg of dichloromethane into the glass-lined main reaction kettle, stir evenly, then use 21% sodium hydroxide solution to adjust the pH of the water phase to 9.1, continue stirring until uniform, let stand to separate the liquid, and separate the water phase.
[0043]Add 100kg KBr into the 1500L secondary reaction kettle, then add 60kg of NaClO, add 550kg of purified water, and stir until completely dissolved. The pH was adjusted to 8.6 with saturated NaHCO3.
[0044] to 10m 3 In the main reactor, add 92.82kg (1.19eq) of 2,2,6,6-tetrameth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com