Preparation method of linagliptin and intermediate thereof
A technology for intermediates and compounds, which is applied in the field of preparation of linagliptin and its intermediates, can solve the problems of difficulty in obtaining compound IV, harsh reaction conditions, high market prices and the like, achieves simple and easy operation, reduced reaction steps, cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
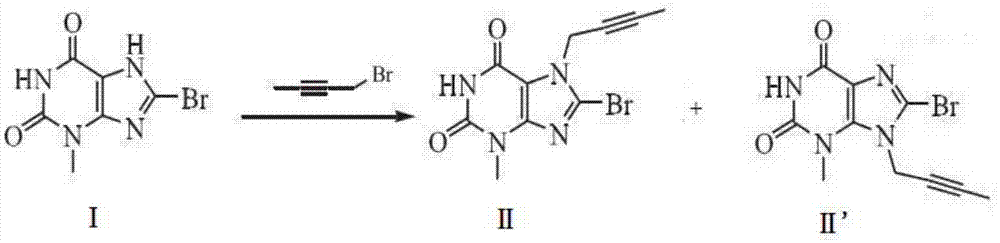
[0047] Embodiment 1: the synthesis of compound II
[0048] First, suspend 8-bromo-3,7-dihydro-3-methyl-1H-purine-2,6-dione (24.5g, 100mmol) in 200ml tetrahydrofuran, then add 2-butyne-1- Alcohol (7.71g, 110mmol), triphenylphosphine (31.5g, 120mmol), at room temperature, diethyl azodicarboxylate (DEAD, 20.9g, 120mmol) was added dropwise to the reaction solution, and the TLC monitoring reaction was complete . Wash with saturated brine, dry with anhydrous sulfuric acid, concentrate the organic phase, recrystallize the residue with ethyl acetate, and dry in vacuo to obtain 27.2 g of compound II with a yield of 90.3% and a purity of 99.2% (HPLC method).
Embodiment 2
[0049] Embodiment 2: the synthesis of compound II
[0050] First suspend 8-bromo-3,7-dihydro-3-methyl-1H-purine-2,6-dione (24.5g, 100mmol) in 200ml N,N-dimethylformamide (DMF) , add 2-butyn-1-alcohol (8.41g, 120mmol) and triphenylphosphine (36.7g, 140mmol) successively, at room temperature, add diisopropyl azodicarboxylate (DIAD, 28.3 g, 140mmol), until the TLC monitoring reaction is complete. Purified water was added for crystallization, and the obtained solid was recrystallized with ethyl acetate and dried in vacuo to obtain 28.3 g of compound II with a yield of 94.8% and a purity of 99.5% (HPLC method).
Embodiment 3
[0051] Embodiment 3: the synthesis of compound II
[0052] First, suspend 8-bromo-3,7-dihydro-3-methyl-1H-purine-2,6-dione (24.5g, 100mmol) in 200ml of dichloromethane, then add 2-butyne- 1-alcohol (7.71g, 110mmol), triphenylphosphine (31.5g, 120mmol), and azodicarbonamide (TMAD, 14g, 120mmol) were added dropwise to the reaction solution at room temperature, and the reaction was completed by TLC monitoring. Wash with saturated brine, dry with anhydrous sulfuric acid, concentrate the organic phase, recrystallize the residue with ethyl acetate, and dry in vacuo to obtain 27.7 g of compound II with a yield of 92.8% and a purity of 99.4% (HPLC method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com