Medicine composition for treating osteoarthritis and preparing technology thereof
A technology for osteoarthritis and composition, applied in the field of pharmaceutical composition for treating osteoarthritis and its preparation process, can solve the problems of weak curative effect and poor stability of oral liquid, and achieve good therapeutic effect and good anti-free radical and antioxidant activity, enhance the effect of human immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The pharmaceutical composition for the treatment of osteoarthritis of the present embodiment is prepared by the following process:
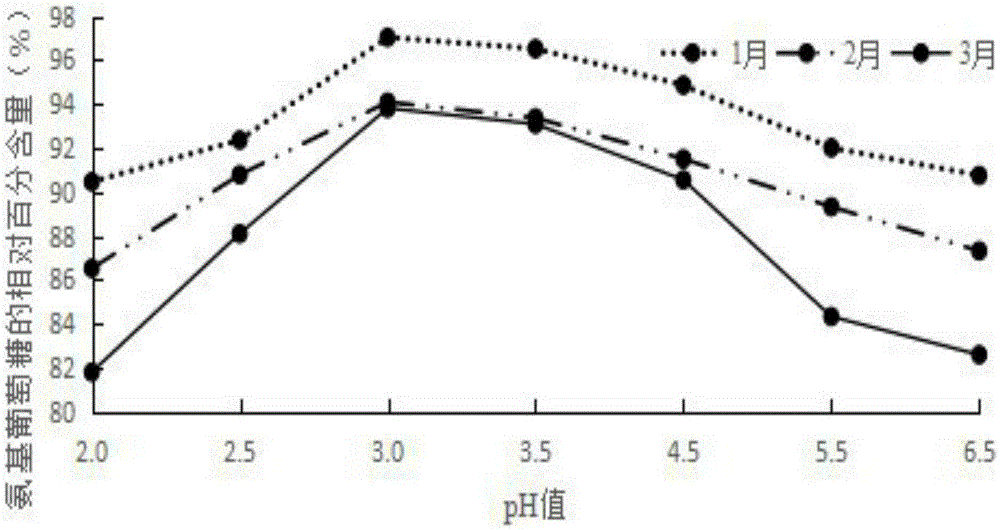
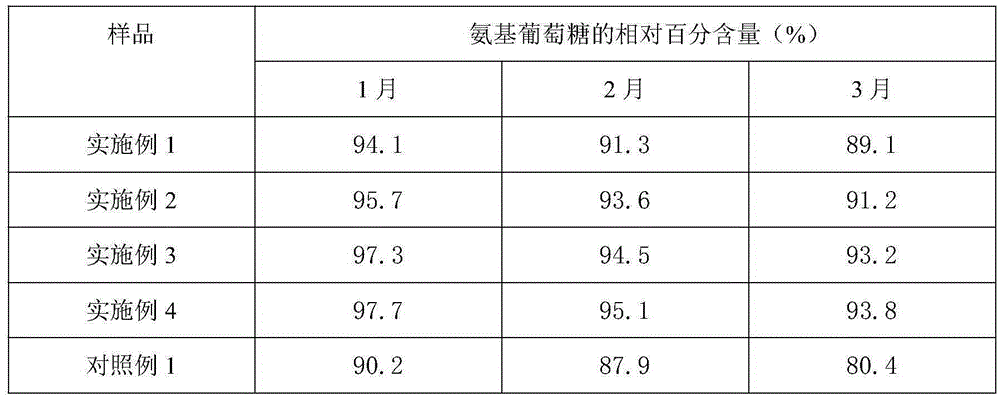
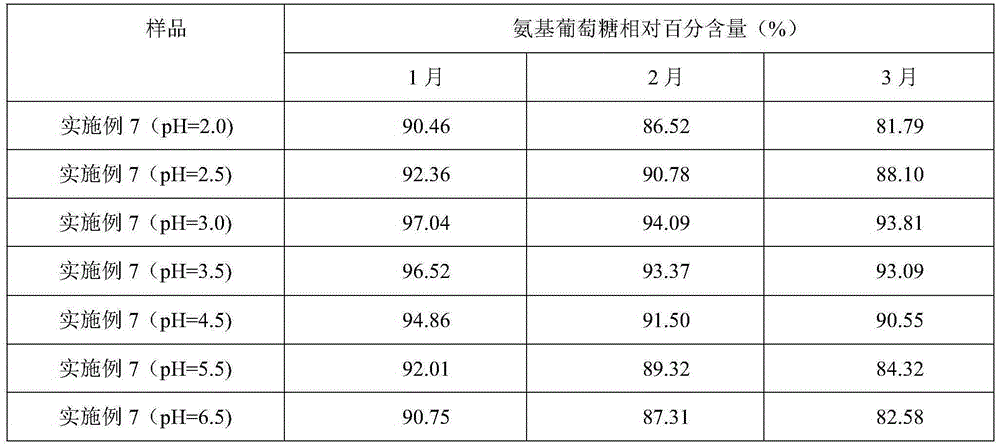
[0037] Weigh raw materials: 25g glucosamine, 6g chondroitin sulfate, 4g taurine, 0.4g aspartame, 0.2g potassium phenylpropionate and 0.1g caramel color, and add the above-mentioned raw materials into 260g deionized water in turn , mix well to obtain an aqueous solution, and use a pH regulator to adjust the pH of the aqueous solution to 3. The aqueous solution with a pH of 3 is firstly filtered through a filter to remove various foreign substances brought in during the weighing and configuration process, and then finely filtered with a 0.22 μm microporous membrane to remove particles and bacteria to obtain a clear liquid, which is obtained after fine filtration liquid medicine. The aseptic filling process is adopted to fill the obtained medicinal liquid into a bottle, and to sterilize the filled bottled oral liquid to kill all the microorg...
Embodiment 2
[0039] The pharmaceutical composition for the treatment of osteoarthritis of the present embodiment is prepared by the following process:
[0040] Weigh raw materials: 35g glucosamine, 10g chondroitin sulfate, 7.5g taurine, 0.8g aspartame, 0.5g potassium phenylpropionate and 0.2g caramel color, and add the above-mentioned raw materials into 380g deionized In water, mix well to obtain an aqueous solution, and use a pH regulator to adjust the pH of the aqueous solution to 3. The aqueous solution with a pH of 3 is firstly filtered through a filter to remove various foreign substances brought in during the weighing and configuration process, and then finely filtered with a 0.22 μm microporous membrane to remove particles and bacteria to obtain a clear liquid, which is obtained after fine filtration liquid medicine. The aseptic filling process is adopted to fill the obtained medicinal liquid into a bottle, and to sterilize the filled bottled oral liquid to kill all the microorgani...
Embodiment 3
[0042] The pharmaceutical composition for the treatment of osteoarthritis of the present embodiment is prepared by the following process:
[0043] Weigh raw materials: 40g glucosamine, 15g chondroitin sulfate, 12g taurine, 1.2g aspartame, 0.8g potassium phenylpropionate and 0.4g caramel coloring, and add the above-mentioned raw materials into 500g deionized water in turn , mix well to obtain an aqueous solution, and use a pH regulator to adjust the pH of the aqueous solution to 3. The aqueous solution with a pH of 3 is firstly filtered through a filter to remove various foreign substances brought in during the weighing and configuration process, and then finely filtered with a 0.22 μm microporous membrane to remove particles and bacteria to obtain a clear liquid, which is obtained after fine filtration liquid medicine. The aseptic filling process is adopted to fill the obtained medicinal liquid into a bottle, and to sterilize the filled bottled oral liquid to kill all the mic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com