Preparation and Application of Phosphorescent Iridium Complexes with Mitochondrial Targeting Function
A phosphorescent iridium complex, iridium complex technology, applied in indium organic compounds, platinum group organic compounds, compounds containing periodic table Group 8/9/10/18 elements, etc., can solve unfavorable synthesis and preparation, exploration. Needle structure is complex and other problems, to achieve the effect of good biocompatibility, improved signal-to-noise ratio, and deep tissue penetration depth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of auxiliary ligand containing triphenylphosphine
[0026]
[0027] Preparation of Compound 2: Compound 1 (26mmol) and sodium methoxide solution were stirred and reacted at room temperature for 22 hours, then ammonium chloride (28mmol) was added to continue the reaction for 6 hours, unreacted ammonium chloride was removed by filtration, and the filtrate was spin-dried to remove methanol solvent , to obtain a light yellow solid powder, and then washed three times with ether to remove unreacted compound 1, and then recrystallized using a mixed solvent of ethanol-ether to obtain compound 2 as a white product. Yield: 83%. 1 H NMR (400MHz, DMSO) δ = 9.69 (s, 4H), 8.85–8.73 (m, 1H), 8.45 (d, J = 8.0Hz, 1H), 8.14 (td, J = 7.8, 1.7Hz, 1H) ,7.85–7.71(m,1H). 13C NMR (101 MHz, DMSO) δ = 162.67, 150.33, 144.37, 138.76, 129.01, 124.00.
[0028] serial number
[0029] Preparation of Compound 3: Compound 2 (5 mmol) and ethyl acetoacetate (5 mmol)...
Embodiment 2
[0036] Embodiment 2: Contain triphenylphosphorus iridium complex Ir-P(Ph) 3 (7) Preparation
[0037]
[0038] Compound Ir-P(Ph)3 Preparation of (7): Mix compound 5 (0.14mmol) and iridium dichloro bridge (0.06mmol) in a mixed solvent of dichloromethane (3mL) and methanol (1mL), stir and reflux overnight at 55°C under a nitrogen atmosphere, and then The reaction solution was cooled to room temperature, and KPF was added thereto 6 (1.4mmol) continued to stir for 4 hours, and after the reaction was completed, the organic solvent was removed by rotary evaporation, and the obtained solid was purified through a column to obtain a red powdery solid Ir-P(Ph) 3 (7). Yield 36%. 1 H NMR (400MHz, DMSO) δ8.43 (d, J = 8.1Hz, 1H), 8.11 (dt, J = 10.7, 5.4Hz, 3H), 8.03–7.95 (m, 2H), 7.93–7.70 (m, 22H),7.36–7.28(m,2H),7.23–6.97(m,14H),6.96–6.88(m,3H),6.86–6.70(m,9H),6.52(dd,J=6.9,4.4Hz, 2H), 4.37 (s, 2H), 3.56 (s, 2H), 1.99 (s, 3H), 1.50 (dd, J = 60.1, 31.4 Hz, 8H).
[0039] se...
Embodiment 3
[0040] Embodiment 3: Iridium complex Ir-P(Ph) 3 (7) Absorption and emission spectrum test
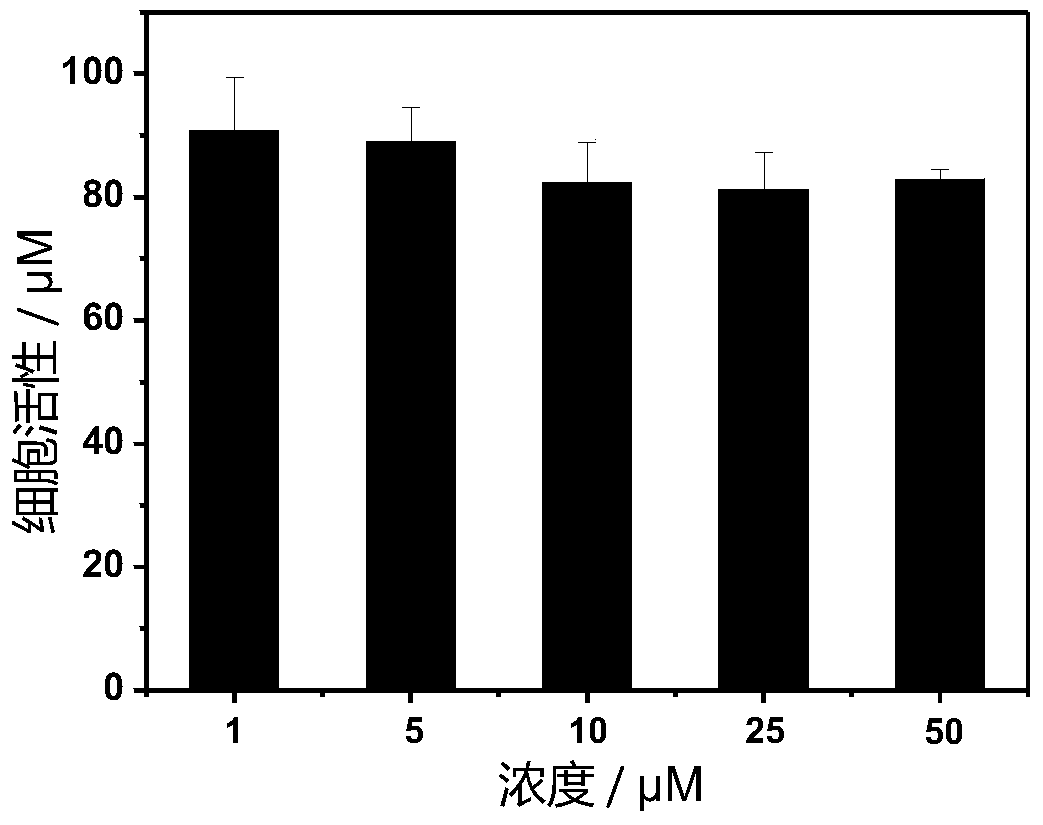
[0041] The spectrum test concentration adopted in the present invention is 10 μM, the test solvent is PBS solution mixed with 1% DMSO, and when the emission spectrum is measured, the excitation wavelength is 405 nm.
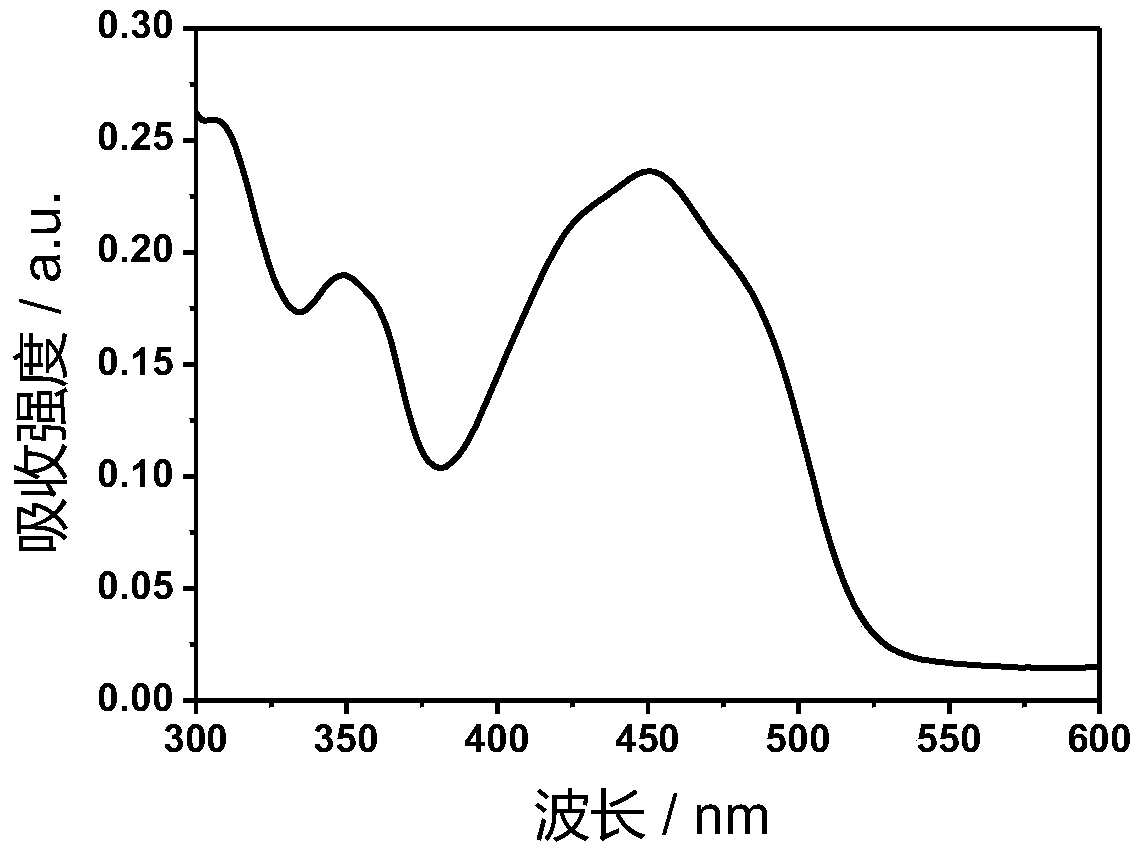
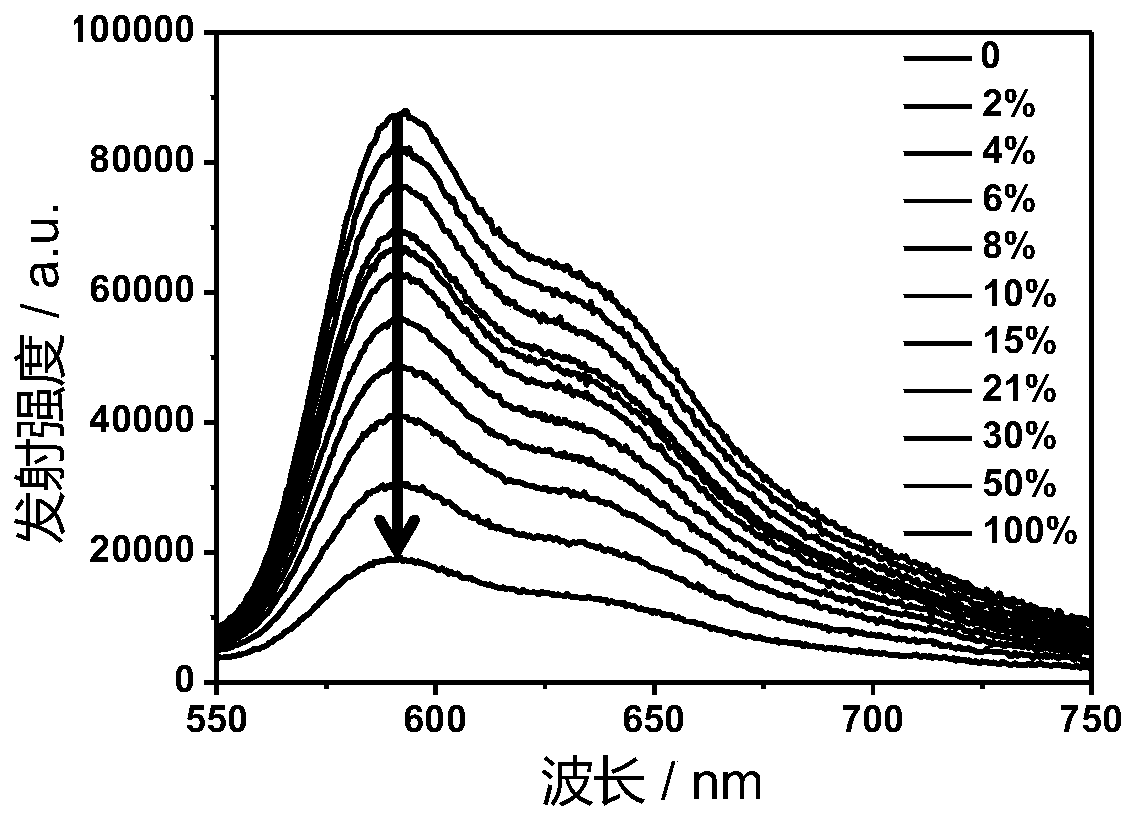
[0042] Ir-P(ph) 3 The absorption and emission spectra of the figure 1 with figure 2 shown. The complex exhibits strong absorption at 250-380nm in the ultraviolet region and 400-500nm in the visible blue light region. In particular, the complex can be excited by visible light, which greatly reduces the damage to cells caused by the excitation light source in cell imaging experiments. Its emission is wide, and the emission peak is located at 602nm. Red light emission increases the penetration depth of biological tissue, making it more suitable for biological imaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com