Preparation and application of water-soluble porphyrin complex
A porphyrin complex, water-soluble technology, applied in the field of preparation of water-soluble porphyrin complexes, can solve the problems of limited depth of light penetrating tissue, limited PDT treatment application, long residence time, etc., and achieves good biocompatibility , the effect of long emission lifetime, simple chemical structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the preparation of polyfluorene
[0025]
[0026] Preparation of Compound 2: Add 1 (3.0 mmol), 1,6-dibromohexane (27.0 mmol), and tetrabutylammonium bromide (0.3 mmol) into a reaction flask, vacuumize, and add 200 mL KOH saturated solution. Stir at 75°C for 1 h, cool down to room temperature, extract with dichloromethane, distill off 1,6-dibromohexane under reduced pressure, load the sample by dry method, and purify by column chromatography to obtain 2. Yield: 83%. 1 HNMR (400MHz, CDCl 3 )δ7.54(s,1H),7.52(s,1H),7.47(d,J=1.7Hz,1H),7.45(d,J=1.7Hz,1H),7.43(d,J=1.6Hz, 2H),5.30(s,2H),3.30(t,J=6.8Hz,4H),1.98–1.86(m,4H),1.74–1.61(m,4H),1.28–1.02(m,10H),0.65 –0.52(m,4H).
[0027] Preparation of Compound 4: Add 3 (3.0 mmol), 1,6-dibromohexane (27.0 mmol), and tetrabutylammonium bromide (0.3 mmol) into a reaction flask, vacuumize, and add 200 mL KOH saturated solution. Stir at 75°C for 1 h, cool down to room temperature, extract with dichloromethane, distill...
Embodiment 2
[0034] Embodiment 2: the preparation of water-soluble porphyrin complex
[0035]
[0036] Preparation of compound 12: Add 11 (2.5mmol) into the reaction flask, vacuumize, add dichloromethane (250mL), blow nitrogen for 20min, add pyrrole (2.5mmol), boron trifluoride diethyl ether (0.8mmol), normal temperature Stir for 3h, then add DDQ (2.0mmol), continue the reaction for 1h, filter with suction, spin off the DCM in the filtrate under reduced pressure, and purify by column chromatography to obtain 12. Yield 15%. 1 H NMR (400MHz, CDCl 3 )δ8.82(s,8H),8.21(q,J=7.9Hz,8H),7.52(s,4H),6.98(d,J=14.8Hz,2H),5.30(s,2H),1.53( d, J=21.4Hz, 48H), -2.82(s, 2H).
[0037] The preparation of compound 13: 12 (1mmol), 10 (3mmol), Pd[P(Ph) 3 ] 4 (0.01mmol) into the reaction flask, vacuumize, add toluene (150mL), ethanol (20mL), potassium carbonate solution (40mL, 2mol / L), stir at 85°C for 2h, cool down to room temperature, extract with dichloromethane, Dry loading and column chromatography ...
Embodiment 3
[0040] Embodiment 3: Absorption and emission spectrum test of porphyrin complex
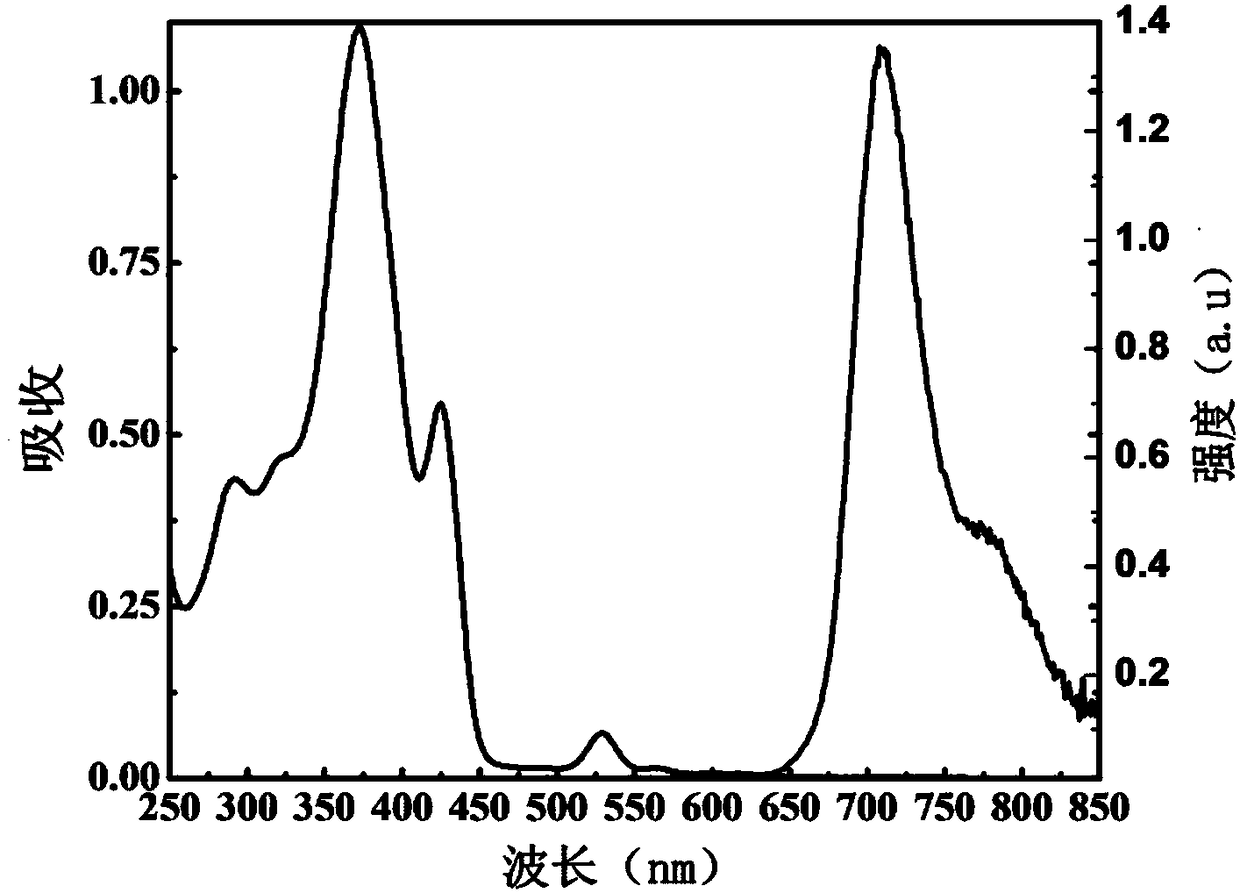
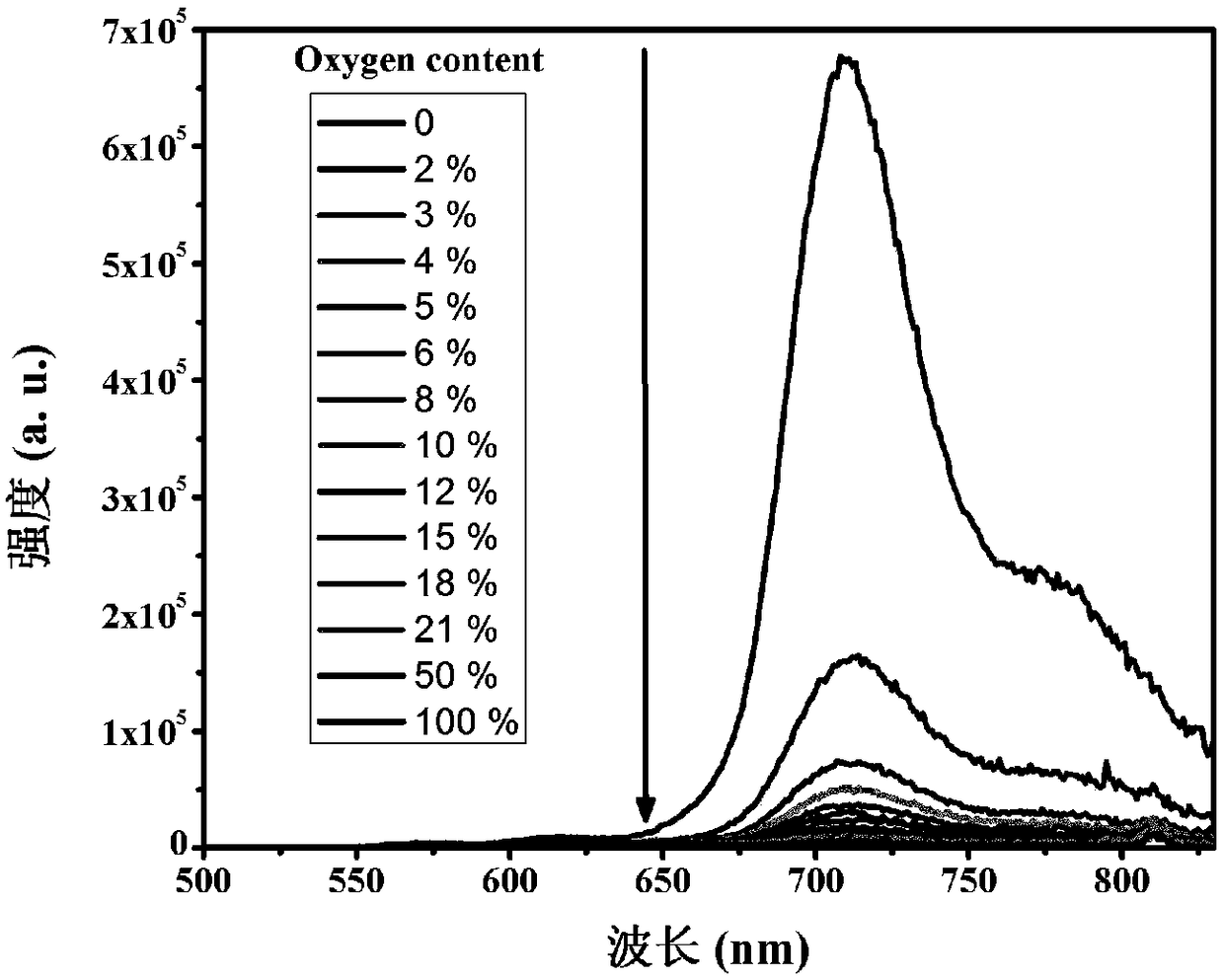
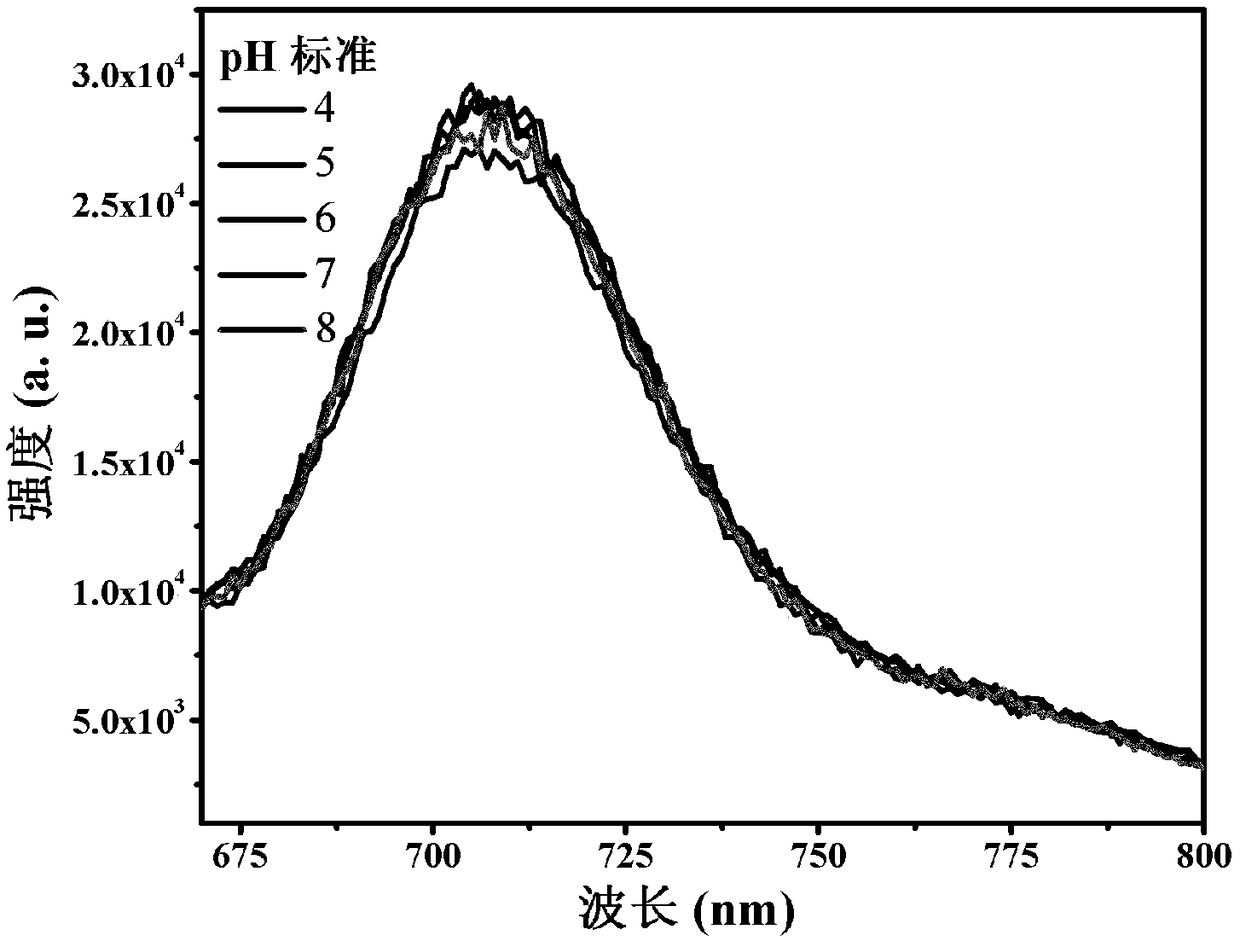
[0041] The spectrum test concentration adopted in the present invention is 10 μM, the test solvent is PBS solution mixed with 1% DMSO, and when the emission spectrum is measured, the excitation wavelength is 520 nm. The absorption and emission spectra of porphyrin as figure 1 shown. The complex exhibits strong absorption at 320-450nm in the ultraviolet region and 500-550nm in the visible light region. In particular, the complex can be excited by visible light, which greatly reduces the damage to cells caused by the excitation light source in cell imaging experiments. Its emission is wide, and the emission peak is located at 710nm. Red light emission increases the penetration depth of biological tissue, making it more suitable for biological imaging. figure 2 It is the emission spectrum of porphyrin under different oxygen concentrations, which shows that the emission decreases gradually with th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com