Injectable antibacterial bone cement
An antibacterial bone and injection technology, applied in medical science, prosthesis, etc., can solve problems such as hindering bacterial DNA replication, bacterial DNA structural denaturation, and bacterial death, and achieve good biocompatibility, degradability, and injectability Good, obvious antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Calcium sulfate 90%, hydroxyapatite 8%, sodium hypophosphite 2%; 2ml concentration is 500mmol / L silver nitrate aqueous solution.
[0048] Take by weighing 4.5g calcium sulfate (calcium sulfate dihydrate 1%), 0.4g hydroxyapatite and 0.1g sodium hypophosphite at room temperature, mix homogeneously; It is the silver nitrate aqueous solution of 500mmol / L that powder is added to 2ml concentration, Mix and stir for 1 minute, and the injectable time is about 5.5 minutes.
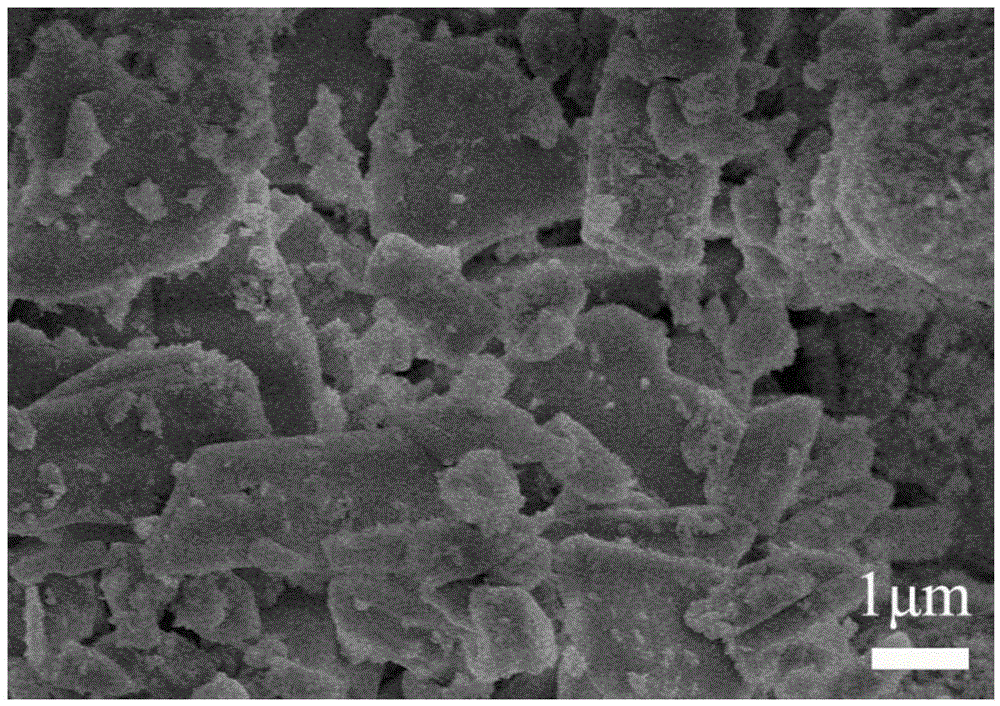
[0049] Place the cured bone cement in the SBF solution for 7 days, dry the cured sample and observe it with a scanning electron microscope, as shown in the attached figure 1 As shown, the cured bone cement is mainly lamellar calcium sulfate dihydrate, and hydroxyapatite particles are evenly distributed on the surface of the lamellar object.
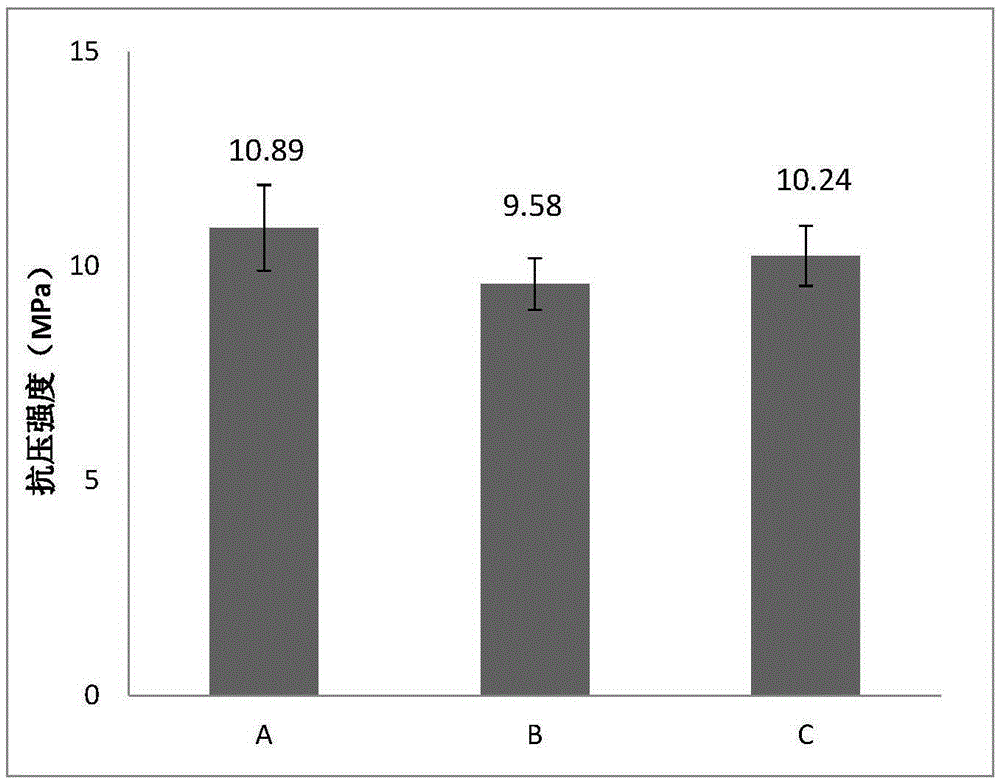
[0050] The bone cement was cured in an environment of 37°C and 100% relative humidity, and the compressive strength test was performed on the bone cement sample cured ...
Embodiment 2
[0052]Calcium sulfate 70%, hydroxyapatite 29%, sodium hypophosphite 1%; 3.34ml of silver nitrate aqueous solution with a concentration of 10mmol / L.
[0053] Take by weighing 3.5g calcium sulfate (calcium sulfate dihydrate 10%), 1.45g hydroxyapatite and 0.05g sodium hypophosphite at room temperature, mix uniformly; Add the powder body to 3.34ml concentration and be the silver nitrate aqueous solution of 10mmol / L , mix and stir for 2 minutes, and the injectable time is about 6 minutes.
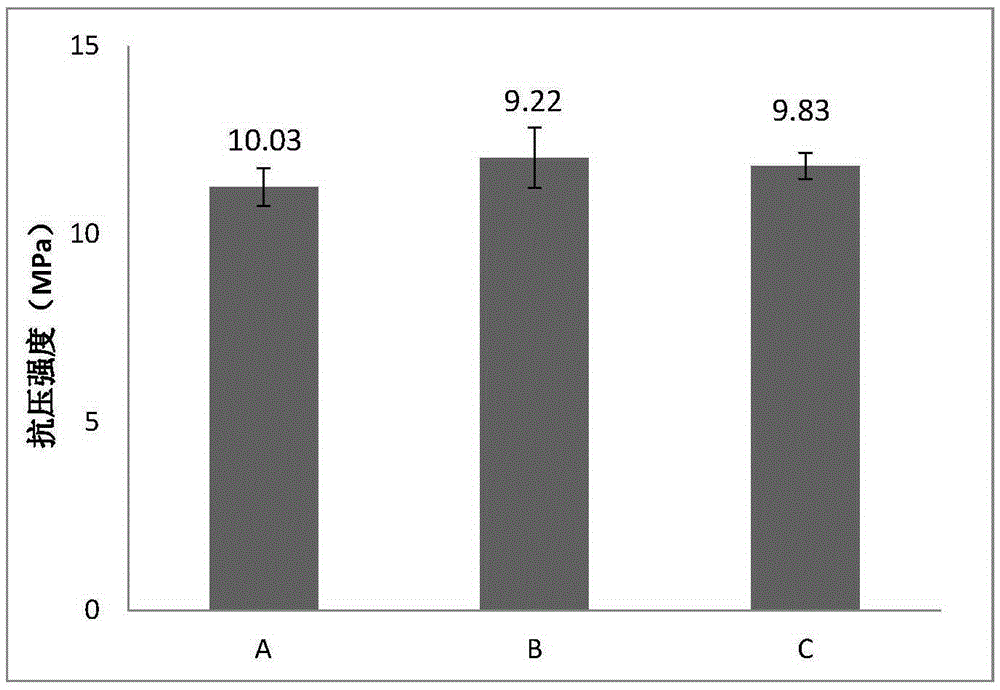
[0054] The bone cement was cured in an environment of 37°C and 100% relative humidity, and the compressive strength test was performed on the bone cement sample cured for 7 days. The compressive strength test sample was cylindrical (Φ6mm×12mm), and the compressive strength The average value is 9.58Mpa (as attached figure 2 -B shown).
[0055] The bone cement is made into discs (Φ6mm×3mm), and antibacterial experiments are carried out on it. It can be seen that the antibacterial bone cement ha...
Embodiment 3
[0057] Calcium sulfate 80%, hydroxyapatite 18.5%, sodium hypophosphite 1.5%; 2.5ml of silver nitrate aqueous solution with a concentration of 100mmol / L.
[0058] Take by weighing 4g calcium sulfate (calcium sulfate dihydrate 5%), 0.925g hydroxyapatite and 0.075g sodium hypophosphite at room temperature, mix homogeneously; Powder is added to 2.5ml concentration and is the silver nitrate aqueous solution of 100mmol / L, Mix and stir for 1.5 minutes, and the injectable time is about 6.5 minutes.
[0059] The bone cement was cured in an environment of 37°C and 100% relative humidity, and the compressive strength test was performed on the bone cement sample cured for 7 days. The compressive strength test sample was cylindrical (Φ6mm×12mm), and the compressive strength The average value is 10.24Mpa (as attached figure 2 -C shown).
[0060] The bone cement is made into discs (Φ6mm×3mm), and antibacterial experiments are carried out on it. It can be seen that the antibacterial bone c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size distribution | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com