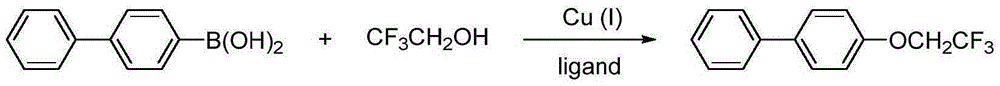Method for preparing aryl trifluoroethoxyl ether
A technology for aryl trifluoroethoxy ethers and compounds, which is applied in the field of synthesis of trifluoroethoxy ether compounds, can solve the problems of unsuitability for industrial applications, narrow substrate universality, and high cost, and achieve a synthetic method Simple and efficient, wide substrate applicability, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Oxidative Coupling Reaction of 4-Biphenylboronic Acid and Trifluoroethanol Catalyzed by Monovalent Copper Salt Using Air as Oxidant.
[0039]
[0040] Using dichloromethane (5ml) as a solvent, add 4-biphenylboronic acid (0.5mmol), trifluoroethanol (1.0mmol) and triethylamine (1.0mmol) under the catalysis of monovalent copper salt CuTc (0.05mmol), air Stir at room temperature under atmosphere for 18 hours. pass 19 FNMR followed the reaction until the reaction was complete. After filtering, removing the solvent, and separating by column chromatography, the corresponding aryl trifluoroethoxy ether compound was obtained. The yield was 45%. The relevant data are as follows: 1 HNMR (400MHz, CDCl 3 )δppm7.56(d, J=7.2Hz, 4H), 7.43(t, J=7.6Hz, 2H), 7.34(t, J=7.2Hz, 1H), 7.07(d, J=8.4Hz, 2H) ,4.40(q,J=8.2Hz,2H). 13 CNMR (100MHz, CDCl 3 )δppm157.0, 140.4, 135.8, 128.9, 128.4, 127.1, 126.9, 123.5 (q, J=278.0Hz), 115.3, 66.0 (q, J=35.4Hz). 19 FNMR (376MHz, CDCl 3 )δppm-...
Embodiment 2
[0042] Oxidative Coupling Reaction of 4-Biphenylboronic Acid and Trifluoroethanol Catalyzed by Divalent Copper Salt Using Air as Oxidant.
[0043]
[0044] With dichloromethane (5ml) as solvent, add 4-biphenylboronic acid (0.5mmol), trifluoroethanol (1.0mmol) and pyridine (1.0mmol) in divalent copper salt Cu(OAc) 2 (0.05 mmol) catalyzed, stirred at room temperature under air atmosphere for 18 hours. pass 19 FNMR followed the reaction until the reaction was complete. After filtering, removing the solvent, and separating by column chromatography, the corresponding aryl trifluoroethoxy ether compound was obtained with a yield of 55%.
Embodiment 3
[0046] Oxidative Coupling Reaction of 4-Biphenylboronic Acid and Trifluoroethanol Catalyzed by Divalent Copper Salt Using Silver Carbonate as Oxidant.
[0047]
[0048] With dichloromethane (5ml) as solvent, add 4-biphenylboronic acid (0.5mmol), trifluoroethanol (1.0mmol), silver carbonate (1.0mmol) and pyridine (1.0mmol) in divalent copper salt Cu(OAc) 2 (0.05 mmol) catalyzed, stirred at room temperature under nitrogen atmosphere for 18 hours. pass 19 FNMR followed the reaction until the reaction was complete. After filtering and removing the solvent, the corresponding aryl trifluoroethoxy ether compound was obtained by column chromatography separation with a yield of 87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com