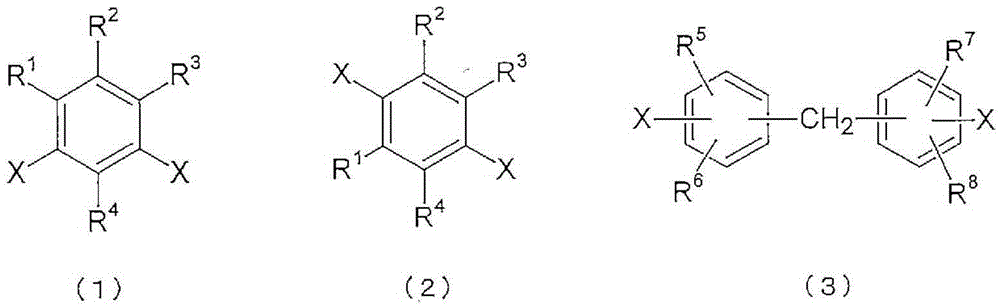
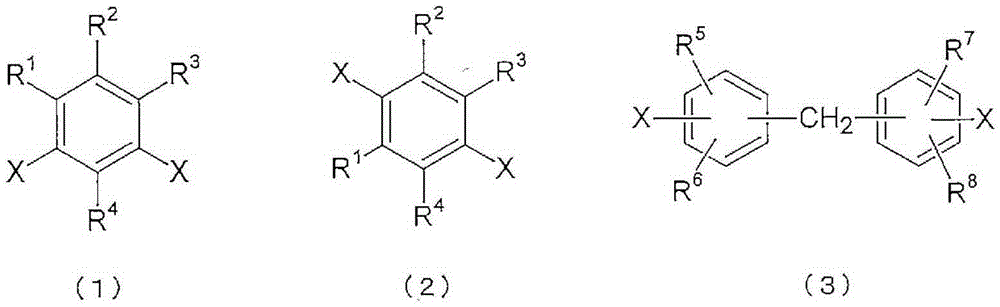
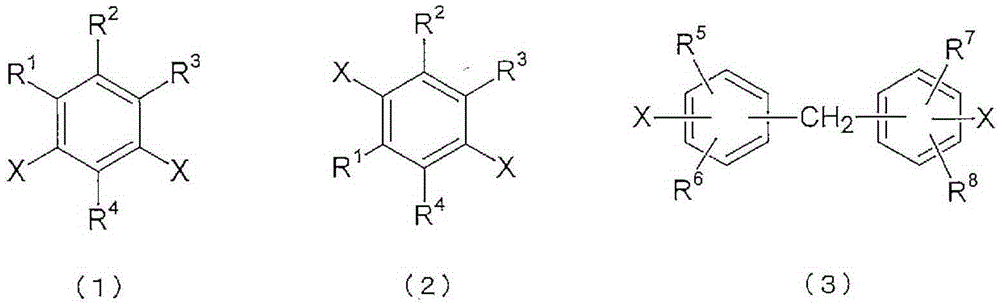
Polyimide copolymer oligomer, polyimide copolymer, and method for producing each of same
A technology of polyimide copolymer and manufacturing method, which is applied in the direction of coating, adhesive, etc., can solve the problems of difficult handling and storage, and achieve the effect of satisfying solvent solubility and excellent practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] 3,3',4,4'-Biphenyltetracarboxylate was added to a 500 mL detachable 4-neck flask equipped with a stainless steel anchor stirrer, a nitrogen gas introduction tube, and a Dean-Stark apparatus (Dean-Starkapparatus). Acid dianhydride (BPDA) 66.52g (0.225 mol), diethyltoluenediamine (DETDA) 32.09g (0.18 mol), N-methyl-2-pyrrolidone (NMP) 100g, pyridine 3.56g, toluene 50g, the Nitrogen replacement was carried out in the reaction system. After dissolving BPDA by stirring at 80° C. for 30 minutes under a nitrogen stream, the temperature was raised to 180° C., and heating and stirring were performed for 2 hours. Water produced by the reaction was removed outside the reaction system by azeotropic distillation with toluene.
[0089] Next, it cooled to 120 degreeC, pDADE9.01g (0.045 mol), and NMP84.22g were added and stirred for 5 minutes, it heated up to 180 degreeC, and it reacted for 6 hours, heating and stirring. Water produced during the reaction was removed outside the reac...
Embodiment 2
[0093] BPDA 41.49g (0.141 mol), DETDA 33.52g (0.188 mol), NMP 84.89g, pyridine 3.72g, and toluene 50g were added to the same apparatus as in Example 1, and the reaction system was replaced with nitrogen. After dissolving BPDA by stirring at 80° C. for 30 minutes under a nitrogen stream, the temperature was raised to 180° C., and heating and stirring were performed for 2 hours. Water produced by the reaction was removed outside the reaction system by azeotropic distillation with toluene.
[0094] Next, cool to 130°C, add 12.52g (0.047mol) of 1-(4-aminophenyl)-1,3,3-trimethyl-1H-indene-5(or 6)-amine (TMDA) and 50g of NMP And after stirring for 5 minutes, PMDA20.74g (0.094mol) and NMP50g were added, it heated up to 180 degreeC, and it reacted for 6 hours, heating and stirring. Water produced during the reaction was removed outside the reaction system as an azeotropic mixture with toluene and pyridine. After completion|finish of reaction, the polyimide solution of 25 mass % dens...
Embodiment 3
[0098]40.60 g (0.138 mol) of BPDA, 16.40 g (0.092 mol) of DETDA, 83.69 g of NMP, 2.91 g of pyridine, and 50 g of toluene were added to the same apparatus as in Example 1, and the reaction system was replaced with nitrogen. After dissolving BPDA by stirring at 80° C. for 30 minutes under a nitrogen stream, the temperature was raised to 180° C., and heating and stirring were performed for 2 hours. Water produced by the reaction was removed outside the reaction system by azeotropic distillation with toluene.
[0099] Next, cool to 130°C, add 32.06 g (0.092 mol) of 9,9-bis(4-aminophenyl)fluorene (FDA) and 60 g of NMP, stir for 5 minutes, and then add 3,3',4,4'- 16.86 g (0.046 mol) of diphenyl sulfone tetracarboxylic dianhydrides (DSDA), NMP40g, heated up to 180 degreeC, and performed reaction for 6 hours, heating and stirring. Water produced during the reaction was removed outside the reaction system as an azeotropic mixture with toluene and pyridine. After completion|finish of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com