Compound Famotidine sustained release tablets and preparing method thereof
A technology of famotidine and sustained-release tablets, applied in the field of medicine, can solve the problems of leukopenia and increase, and achieve the effects of fast onset, good curative effect and good synergistic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
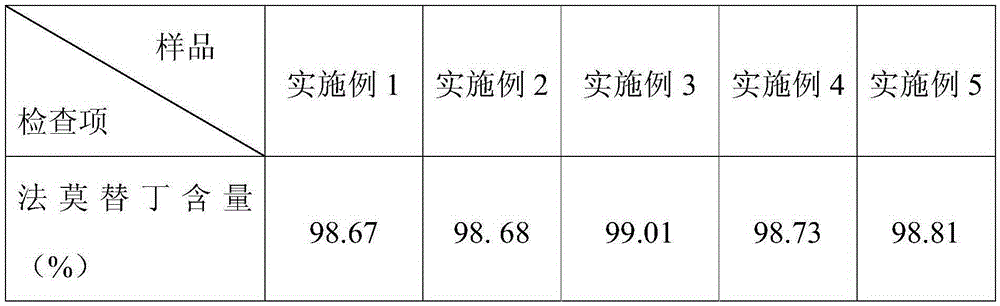
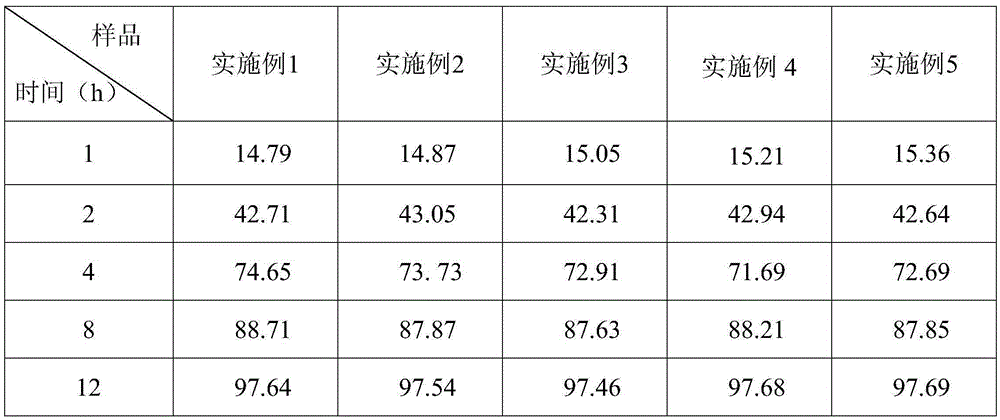
[0022] A compound sustained-release tablet of famotidine, the components and parts by weight of the sustained-release tablet include: 1 part of famotidine, 10 parts of Yuanhu, 12 parts of Ferulicum, 10 parts of aloe, 11 parts of Yu Liren, radish 9 parts of lotus seeds, 10 parts of hollow bubbles, 12 parts of poria cocos, 11 parts of Codonopsis pilosula, 10 parts of licorice, 14 parts of microcrystalline cellulose pellets, 2 parts of polyacrylic resin, 0.3 parts of glyceride, 0.2 parts of hypromellose, starch 5 parts, 2 parts of calcium stearate, 13 parts of sodium carbonate, 1 part of hydroxypropyl cellulose.
[0023] A preparation method for famotidine compound sustained-release tablets, the specific steps comprising:
[0024] 1) raw material preparation: prepare raw materials according to the above-mentioned components and parts by weight of a kind of famotidine compound sustained-release tablet, starch, calcium stearate, sodium carbonate are pulverized respectively and pass...
Embodiment 2
[0031] A compound sustained-release tablet of famotidine, the components and parts by weight of the sustained-release tablet include: 4 parts of famotidine, 19 parts of Yuanhu, 23 parts of ferulicum, 20 parts of aloe, 24 parts of Yu Liren, radish 18 parts of lotus seeds, 22 parts of hollow bubbles, 23 parts of poria cocos, 24 parts of Codonopsis pilosula, 18 parts of licorice, 25 parts of microcrystalline cellulose pellets, 5 parts of polyacrylic resin, 0.7 parts of glyceride, 0.5 parts of hypromellose, starch 14 parts, 7 parts of calcium stearate, 19 parts of sodium carbonate, 4 parts of hydroxypropyl cellulose.
[0032] The preparation method of above-mentioned a kind of famotidine compound sustained-release tablet is the same as embodiment 1.
Embodiment 3
[0034] A compound sustained-release tablet of famotidine, the components and parts by weight of the sustained-release tablet include: 2 parts of famotidine, 13 parts of Yuanhu, 15 parts of Ferulicum, 13 parts of aloe, 16 parts of Yu Liren, radish 12 parts of lotus seeds, 12 parts of hollow bubbles, 15 parts of poria cocos, 16 parts of Codonopsis pilosula, 13 parts of licorice, 17 parts of microcrystalline cellulose pellets, 3 parts of polyacrylic resin, 0.4 parts of glyceride, 0.3 parts of hypromellose, starch 6 parts, 3 parts of calcium stearate, 14 parts of sodium carbonate, 2 parts of hydroxypropyl cellulose.
[0035] The preparation method of above-mentioned a kind of famotidine compound sustained-release tablet is the same as embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com