Amphiphilic oligopeptide drug conjugate
A technology of oligomeric polypeptides and conjugates, which can be used in drug combination, drug delivery, pharmaceutical formulations, etc., can solve the problem of insufficient anti-tumor preparations, and achieve the effects of rich varieties, high yield and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
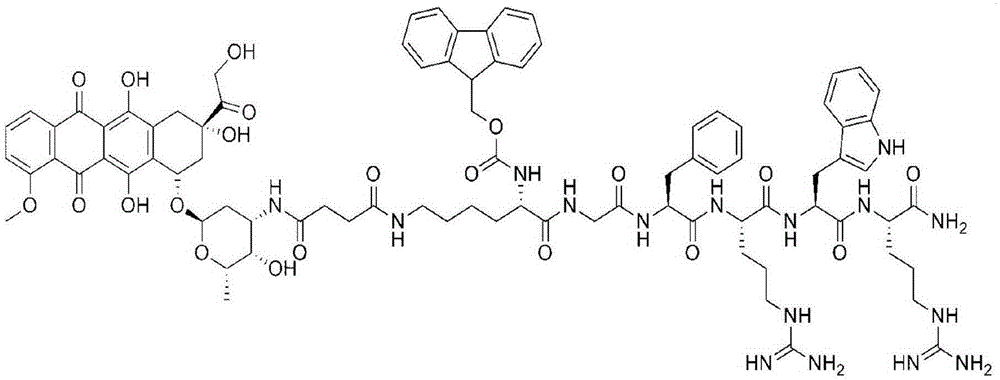
[0029] Embodiment 1: Amphiphilic oligomeric polypeptide drug conjugate (Dox- Fmoc-KGFRWR: doxorubicin-fluorenylmethoxycarbonyl protected lysine-glycine-phenylalanine-arginine-tryptophan-arginine)
[0030] 1. Materials
[0031] Lysine (K) protected with fluorenylmethoxycarbonyl (Fmoc) and 4-methyl-trityl (Mtt), glycine (G) protected with tert-butoxycarbonyl (Boc), phenylpropanoid protected with Boc amino acid, Boc-protected arginine and tryptophan, N,N'-diisopropylcarbodiimide (DIC, 99%), 1-hydroxybenzotriazole (HOBt, 99%), 2- (1H-Benzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU, 99%), N,N-dimethylaminopyridine (DMAP, 99%), 9-fluorenylmethoxycarbonyl (Fmoc, 99%), N,N-diisopropylethylamine (DIEA, 99%), triisopropylsilane (TIS), (3H-1,2,3-triazole [4,5-b]pyridine-3-oxyl)tris-1-pyrrolidinyl hexafluorophosphate (PyAOP), N,N-dimethylformamide (DMF, 99%), pyridine, piperidine , acetic anhydride, ninhydrin, king resin, acetonitrile, trifluoroacetic acid (TFA),...
Embodiment 2
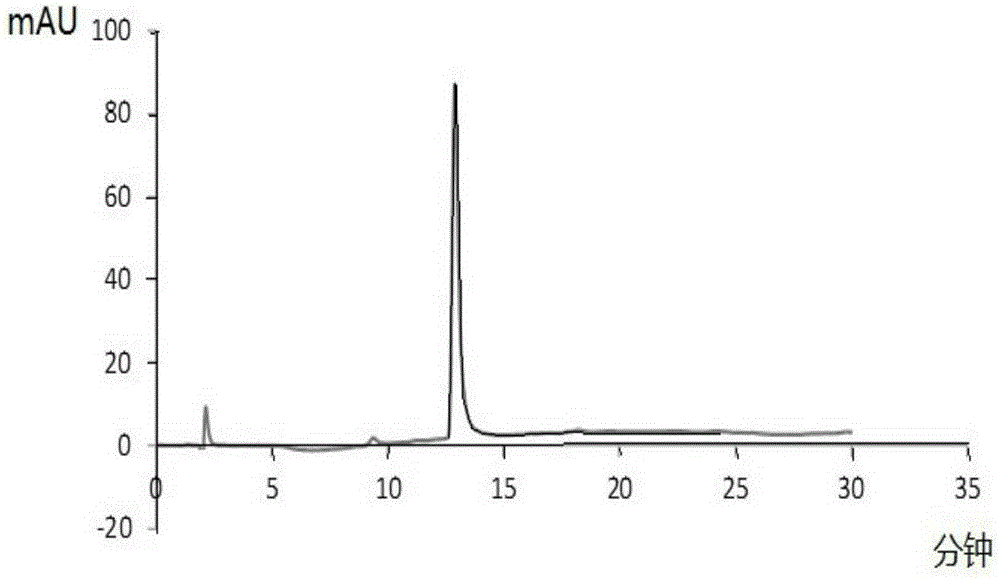
[0039] Embodiment 2: the establishment of the standard curve of Dox-Fmoc-KGFRWR
[0040] Get the Dox-Fmoc-KGFRWR prepared in Example 1 and be formulated with 1mg / ml mother liquor, serially diluted to 5, 10, 50, 100, 250, 500ug / ml, enter high-performance liquid phase (HPLC), record peak area, draw standard curve , see Figure 4 .
Embodiment 3
[0041] Example 3: Transmission Electron Microscopy (TEM) Inspection of Dox-Fmoc-KGFRWR Self-Assembly at Different pH Values
[0042] (1) Hydrochloric acid and sodium hydroxide solution adjust the pH value of the aqueous solution to 3 and 7 respectively, and set aside. Weigh an appropriate amount of Dox-Fmoc-KGFRWR from Example 1 and add it into prepared solutions with different pH values to make a 500uM solution, and let it stand for several days.
[0043] (2) First, drop the prepared sample to be tested on the 400-mesh copper grid, use it for a while, take out the copper grid with tweezers, absorb the excess liquid with filter paper, put the copper grid on the phosphotungstic acid stain for about 90 seconds, and use After taking out the copper mesh with tweezers, suck off the excess liquid with filter paper, place it to dry, prepare for the experiment, and observe its imaging situation with a transmission electron microscope, see Figure 5 , left to right are electron micr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com