Synthesis and fluorescence detection imaging application of phosphorescence iridium complex
A phosphorescent iridium complex and iridium complex technology, applied in the field of amino acid detection, can solve the problems of living tissue damage, insufficient tissue penetration depth, low luminescence quantum efficiency of fluorescent probes, etc., and achieve strong tissue penetration and spontaneous background. The effect of less fluorescence interference and less light damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Iridium complex [Ir(CHO-btiq) 2 (bpy)][PF 6 ]Synthesis:
[0040] (a) Weigh 4mmol (766.4mg) 2-chloroquinoline-3-formaldehyde and 4mmol (712.1mg) benzothiophene-2-boronic acid in a 100mL round bottom flask, add 3 molar equivalents of potassium carbonate, add 40mL volume ratio V / V=1:1 mixed solvent of tetrahydrofuran and water, finally add 8% molar equivalent of tetrakis(triphenylphosphine)palladium, heat and reflux at 70°C for 24 hours in a nitrogen protective atmosphere. After the reaction, the reaction liquid was extracted with dichloromethane, and the aqueous phase was extracted three times with dichloromethane (3×10mL). The organic phases were combined, dried overnight with anhydrous sodium sulfate, and the solvent was removed under reduced pressure. After column chromatography separation, the formula The ligand for II, CHO-tbiq. NMR characterization data: 1 HNMR (400MHz, CDCl 3 )δ=10.55(s,1H),8.82(s,1H),8.23(d,J=8.5Hz,1H),8.01(d,J=8.0Hz,1H),7.98–7.92(m,1H), 7....
Embodiment 2
[0044] Response of iridium complexes to cysteine:
[0045] a) The phosphorescent iridium complex was prepared into a 10 μM dilute solution with a mixed solvent of acetonitrile and water at a volume ratio of V / V=4:1.
[0046] b) Prepare 2 mmol of cysteine aqueous solution.
[0047] c) adding 0-40 equivalents of cysteine aqueous solution to the dilute solution of the iridium complex, and reacting at 37° C. for 5 hours.
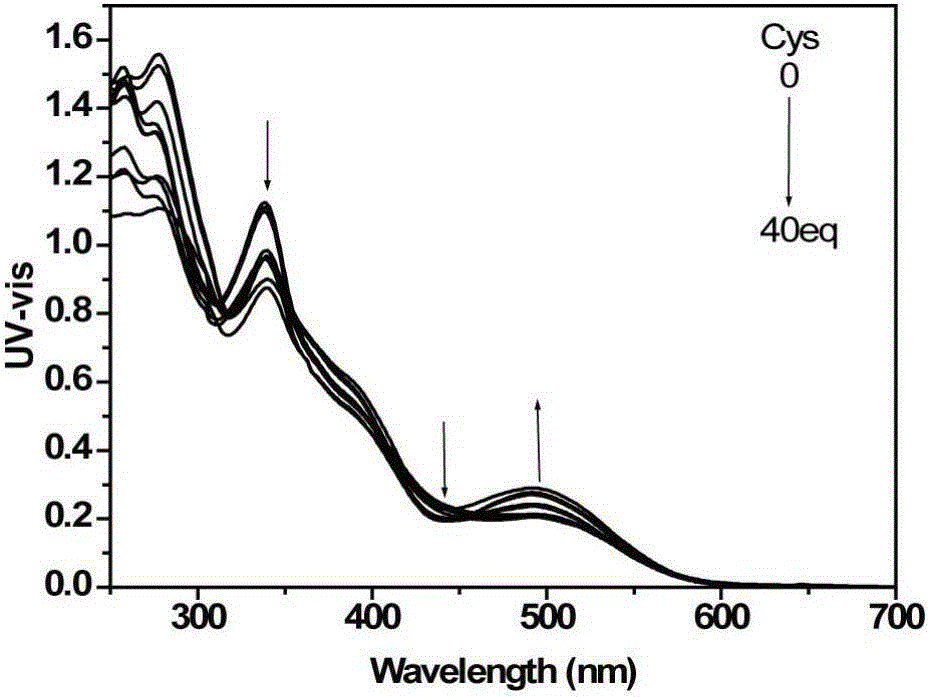
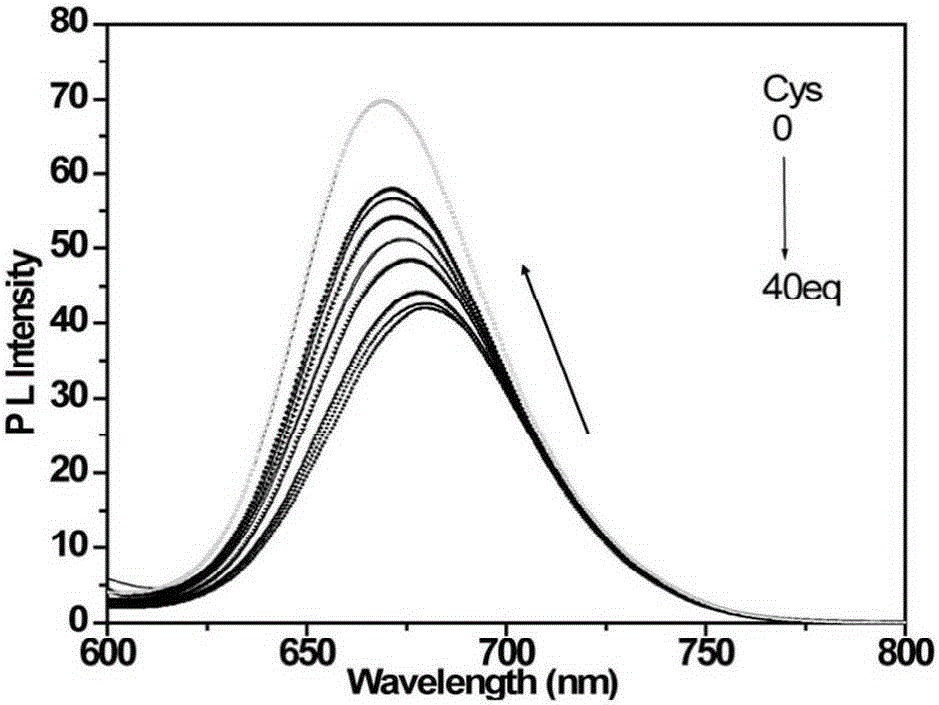
[0048] d) detect the changes of the ultraviolet absorption spectrum and the fluorescence emission spectrum of the solution with an ultraviolet spectrophotometer and a fluorescence spectrophotometer, such as figure 1 with figure 2 shown. With the increase of cysteine addition, the ultraviolet absorption of the iridium complex at 338nm and 480nm gradually weakened, and the ultraviolet absorption at 495nm increased; the fluorescence signal blue shifted with the increase of cysteine addition , and the signal is enhanced. This is because the aldehyde gr...
Embodiment 3
[0050] Response of iridium complexes to homocysteine:
[0051] a) The phosphorescent iridium complex was prepared into a 10 μM dilute solution with a mixed solvent of acetonitrile and water at a volume ratio of V / V=4:1.
[0052] b) Prepare a 2 mmol homocysteine aqueous solution.
[0053] c) adding 0-40 molar equivalent homocysteine aqueous solution to the dilute solution of the iridium complex, and reacting at 37° C. for 5 hours.
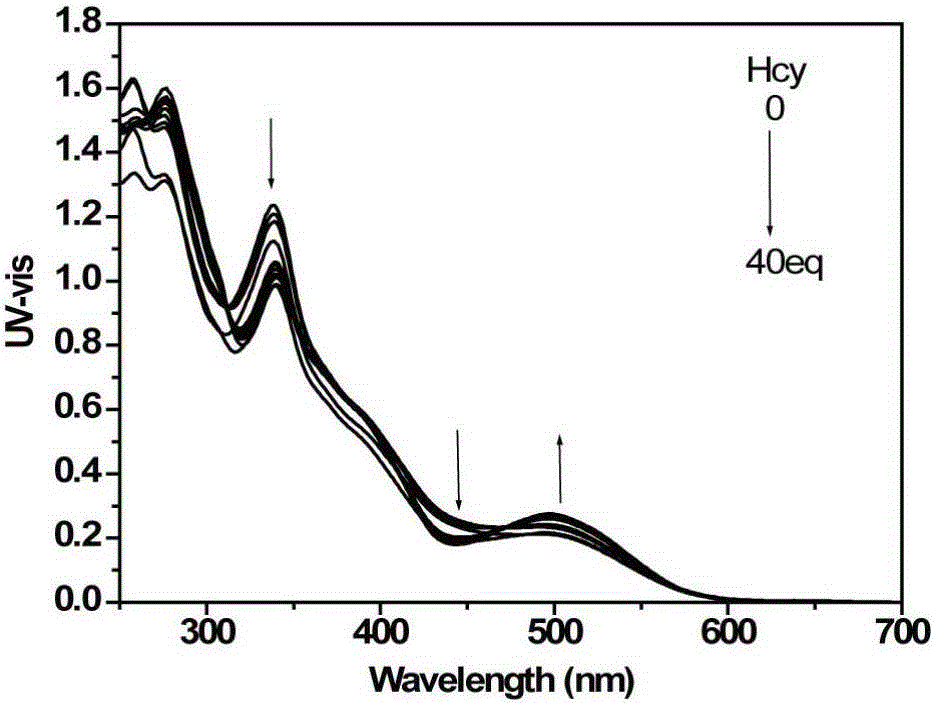
[0054] d) detect the changes of the ultraviolet absorption spectrum and the fluorescence emission spectrum of the solution with an ultraviolet spectrophotometer and a fluorescence spectrophotometer, such as image 3 with Figure 4 shown. With the increase of the addition of cysteine, the ultraviolet absorption of the iridium complex at 338nm and 480nm gradually weakens, and the ultraviolet absorption at 495nm increases; the fluorescence signal blue shifts with the increase of the addition of homocysteine , and the signal is enhanced. This i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com