Z structural domain derivative of staphylococcus protein A and application thereof
A Staphylococcus, structural domain technology, applied in the direction of anti-bacterial immunoglobulin, application, peptide/protein composition, etc., can solve problems such as recurrence, reduction of specific protein A antibodies, and pathogenic bacteria infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0180] The construction of embodiment 1 protein carrier
[0181] According to the sequence of the open reading frame of the sumo gene (a small molecule ubiquitin-like modified protein, used as a fusion tag for recombinant protein expression), the T domain gene mRNA of DT and the protein A gene mRNA in GeneBank, the primers were designed by the principle of overlapping PCR in After the sumo gene, connect the T domain of DT, and then introduce one of the domain Z of protein A, and introduce two mutations in domain Z, F13G / Y14G, and introduce restriction sites NdeI and BamHI at the head and tail , and the introduction of protective bases, connected to PET28. Synthesized by Nanjing GenScript Biotechnology Co., Ltd. according to the primers. The peptide chain amino acid sequence of the specifically designed sumo-DTT-ZFY of the present invention is shown in SEQIDNO: 7; its DNA sequence is shown in SEQIDNO: 8; and the primers are designed and synthesized as follows: P1: SEQIDNO: 14;...
Embodiment 2
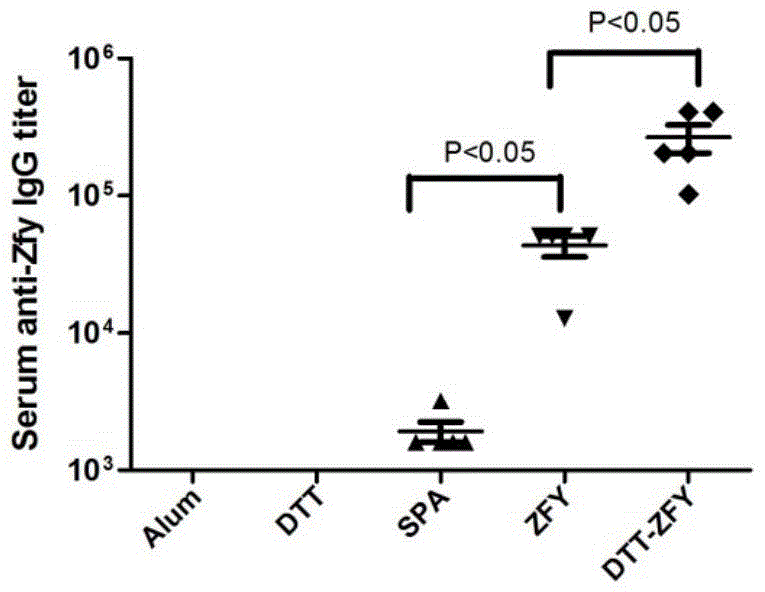
[0197] Example 2 Expression identification and large-scale preparation of protein
[0198] Protein
[0199] A single colony of Escherichia coli containing the recombinant expression plasmid sumo-DTT-ZFY fusion protein was inoculated into 5 mL of LB medium and cultured overnight at 37°C. Then diluted 1:50 into 50 mL of LB medium containing 30 ng / L kanamycin, cultured for about 4 hours to make the OD600 value about 0.6, added IPTG to a final concentration of 1 mM, and took out after induction at 37°C for 4 hours sample.
[0200] Take 50mL of the induced culture, place it on ice for 30min, centrifuge at 12000rpm for 8min, collect the bacteria, discard the supernatant; dissolve and resuspend the precipitate with 1×PBS (5mL / 100mL culture) plus an equal volume of ice-cold sterile water; add 300μg / mL lysozyme, shake gently on ice for 30min, then freeze at -70°C for 12h; melt the sample with running water, add protease inhibitors, ultrasonically break (200W power) in an ice bath, br...
Embodiment 3
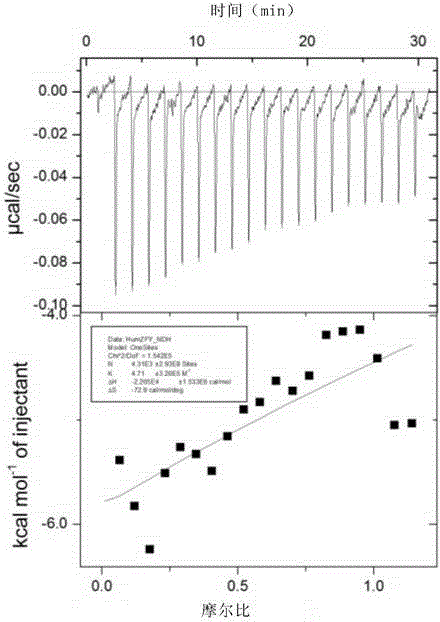
[0207] Embodiment 3 isothermal titration experiment
[0208] By adopting isothermal titration calorimetry method, the change of the binding constant of Z domain of native protein A and ZFY mutant to human and rabbit IgG was determined. If it is necessary to verify that the mutated domain ZFY has almost no binding reaction with IgG, it can be intuitively reflected by the change of its reaction energy. If there is no obvious energy change during the mixing process, it can be considered that there is no binding between the ZFY protein and IgG, and the point mutation transformation of the Z domain is successful; and if the two have obvious energy changes during the mixing process If the energy changes, it can be inferred that the ZFY protein will still react with IgG, and such point mutations have no effect or are incomplete. Through isothermal titration experiments, it is possible to effectively verify whether the transformation of the Z domain of protein A is successful in vitr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com