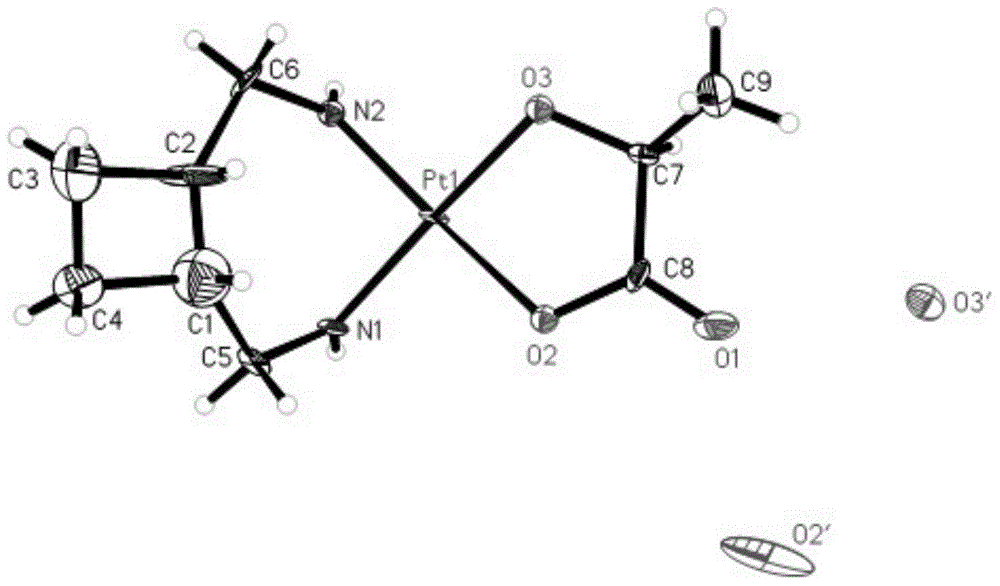
Lobaplatin dihydrate, preparation method and drug application
A lobaplatin and drug technology, applied in the field of lobaplatin dihydrate and its preparation, can solve the problems of poor stability and difficulty in making lobaplatin, achieve good stability, not easy to deliquescence, and improve the quality of drug production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] On the other hand, the present invention provides a method for preparing a new crystal form of lobaplatin that is simple to prepare, easy to operate, and suitable for scale-up production, comprising the following steps:
[0049] Weigh lobaplatin trihydrate in a container, add an organic solvent, suspend and stir at room temperature for 45-50h, filter, wash with ether, and vacuum dry to obtain a white powder, which is lobaplatin dihydrate; the organic solvent is selected from From methyl tert-butyl ether, toluene, diethyl ether, butyl acetate, 1,4-dioxane or n-heptane, the mass volume ratio is lobaplatin trihydrate: organic solvent = 1:15-30.
Embodiment 1
[0052] Example 1: Screening analysis of various crystal forms of lobaplatin
[0053] 1.1 Screening by room temperature volatile crystallization method
[0054] Take 20 mg of lobaplatin trihydrate sample and put it into a 10 ml sample bottle, add 3 ml of absolute ethanol or absolute methanol, after fully dissolving, place it in an environment of 25°C and slowly volatilize to obtain a dry solid, which is then determined by PXRD. The results are shown in Table 2 below:
[0055] Table 2 normal temperature volatilization and crystallization test results
[0056] Numbering
PXRD (possible crystal number)
1-1
Absolute ethanol
B
1-2
B
[0057] The results show that the crystal forms obtained in anhydrous methanol and absolute ethanol are the same crystal form through PXRD diffraction pattern analysis and comparison, and are named as crystal form B.
[0058] 1.2 Screening by suspension crystallization m...
Embodiment 2
[0101] Example 2: Preparation of Lobaplatin Dihydrate Named Crystal Form A
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com