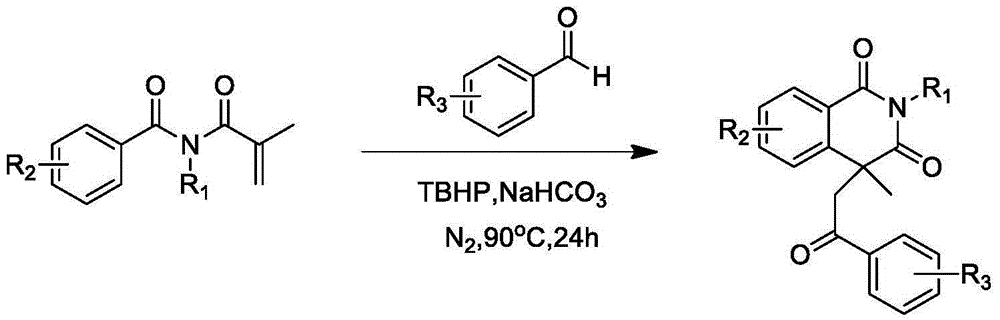
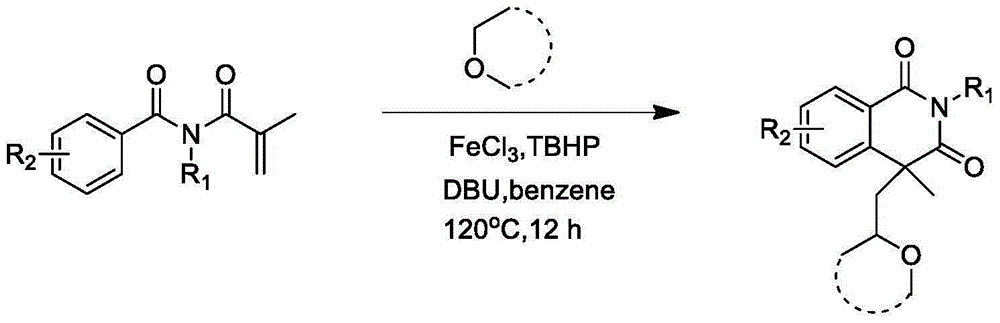
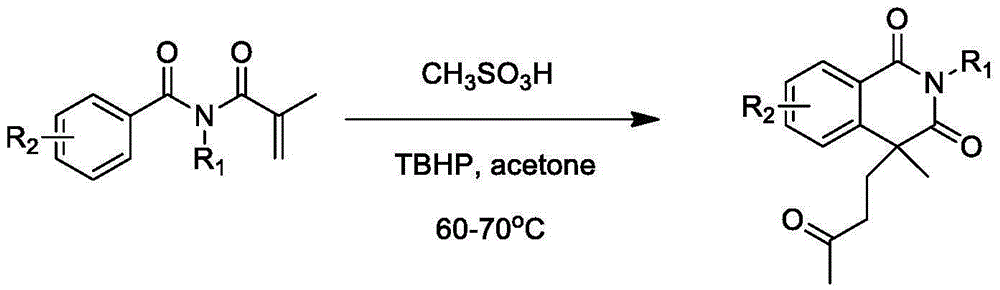
Preparation of 1,3-isoquinoline dione derivative
A technology for isoquinoline dione and derivatives, applied in 1 field, can solve the problem of not many 1,3-isoquinoline dione derivatives, and achieve the effects of simple operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: The preparation method of 2,4-dimethyl-4-(3'-butanone)-1,3-isoquinolinedione, the synthetic route is:
[0022]
[0023] Example 1: The preparation method of 2,4-dimethyl-4-(3'-butanone)-1,3-isoquinolinedione is carried out as follows:
[0024] (1) At room temperature, add N-methyl-N-methacryloylbenzamide (0.203g, 1.0mmol) and methanesulfonic acid (13μL, 0.2mmol) to 6mL of acetone respectively, and add tert Butyl hydroperoxide (500 μL, 3.0 mmol). The mixed system was reacted in an oil bath at 60 to 70°C for 12 hours.
[0025] (2) After the reaction is over, add water to quench. It was extracted twice with ethyl acetate (20 mL×2), washed with saturated brine once, and spin-dried under reduced pressure to remove ethyl acetate. The oily target product (0.145 g, yield 56%) was obtained through column chromatography. 1 HNMR (400MHz): 8.25-8.27 (m, 1H), 7.63-7.68 (m, 1H), 7.42-7.48 (m, 2H), 3.39 (s, 3H), 2.47-2.54 (m, 1H), 2.17- 2.25(m,2H),1.99(s,3H),1.84-1.91(m,1H),1....
Embodiment 2
[0026] Example 2: The preparation method of 4-methyl-2-phenyl-4-(3'-butanone)-1,3-isoquinolinedione, the synthetic route is:
[0027]
[0028] Example 2: The preparation method of 4-methyl-2-phenyl-4-(3'-butanone)-1,3-isoquinolinedione is carried out as follows:
[0029] (1) At room temperature, add N-methacryloyl-N-phenylbenzamide (0.265g, 1.0mmol) and methanesulfonic acid (13μL, 0.2mmol) to 6mL of acetone respectively, and add tert Butyl hydroperoxide (500 μL, 3.0 mmol). The mixed system was reacted in an oil bath at 60 to 70°C for 12 hours.
[0030] (2) After the reaction is over, add water to quench. It was extracted twice with ethyl acetate (20 mL×2), washed with saturated brine once, and spin-dried under reduced pressure to remove ethyl acetate. The target product (0.128 g, yield 40%) was obtained by column chromatography. Melting point: 110-112°C. 1 HNMR (400MHz): 8.26-8.28 (m, 1H), 7.67-7.71 (m, 1H), 7.41-7.50 (m, 5H), 7.17-7.19 (m, 2H), 2.49-2.51 (m, 1H), 2.31-2.37(m,2H...
Embodiment 3
[0031] Example 3: The preparation method of 2,4-dimethyl-4-(3'-pentanone)-1,3-isoquinolinedione, the synthetic route is:
[0032]
[0033] Example 3: The preparation method of 2,4-dimethyl-4-(3'-pentanone)-1,3-isoquinolinedione was carried out as follows:
[0034] (1) At room temperature, add N-methyl N-methacryloyl benzamide (0.203 g, 1.0 mmol) and methanesulfonic acid (13 μL, 0.2 mmol) to 6 mL of 2-butanone, and stir at room temperature Add tert-butyl hydroperoxide (500 μL, 3.0 mmol). The mixed system was reacted for 12 hours in an oil bath at 70 to 80°C.
[0035] (2) After the reaction is over, add water to quench. It was extracted twice with ethyl acetate (20 mL×2), washed with saturated brine once, and spin-dried under reduced pressure to remove ethyl acetate. The oily target product (0.087g, yield 32%) was obtained by column chromatography. 1 HNMR (400MHz): 8.25-8.27 (m, 1H), 7.63-7.67 (m, 1H), 7.42-7.47 (m, 2H), 3.39 (s, 3H), 2.47-2.54 (m, 1H), 2.11 2.30(m,4H),1.81-1.89(m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com