Photocatalyst composition CNB-BiVO4 and preparation method and application thereof
A photocatalyst and composition technology, applied in chemical instruments and methods, water pollutants, light water/sewage treatment, etc., can solve the problem of high cost of raw materials, limited increase in photocatalytic efficiency of modified catalysts, and inability to meet industrial requirements, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] (1) Weigh 10.0g of urea and 5.0mg of sodium tetraphenylborate, put them into a clean and dry small beaker, add 20mL of distilled water, mix and dissolve evenly, and then put the small beaker into an 80°C water bath (preheated to 80°C), evaporate the water to dryness to obtain a solid, move the obtained solid into a crucible, put it into a muffle furnace, and calcinate at 550°C for 2 hours to obtain a CNB sample;
[0080] (2) Weigh 19.4gBi(NO 3 ) 3 ·5H 2 O and 4.68gNH 4 VO 3 Dissolve in 500mL nitric acid solution (2mol / L), mix well, transfer the mixed solution into a 1000mL beaker to obtain an orange solution, then add 7.5g urea to the mixed solution to adjust the pH of the mixed solution value to 7.5,
[0081] The mixed solution was placed in an oil bath magnetic stirrer at 90°C and fully stirred for 24 hours. A bright yellow crystalline solid gradually formed. After the reaction was complete, the resulting solid was suction filtered, and the solid obtained by suct...
Embodiment 2
[0084] The method used in Examples 2-5 is similar to that of Example 1, the only difference is that in step (3), the CNB powder prepared in step 1 and the BiVO powder prepared in step 2 are weighed 4 The weights of the powders were respectively 0.2g and 0.8g (Example 2), 0.5g and 0.5g (Example 3), 0.8g and 0.2g (Example 4), 0.9g and 0.1g (Example 5), respectively.
experiment example 1
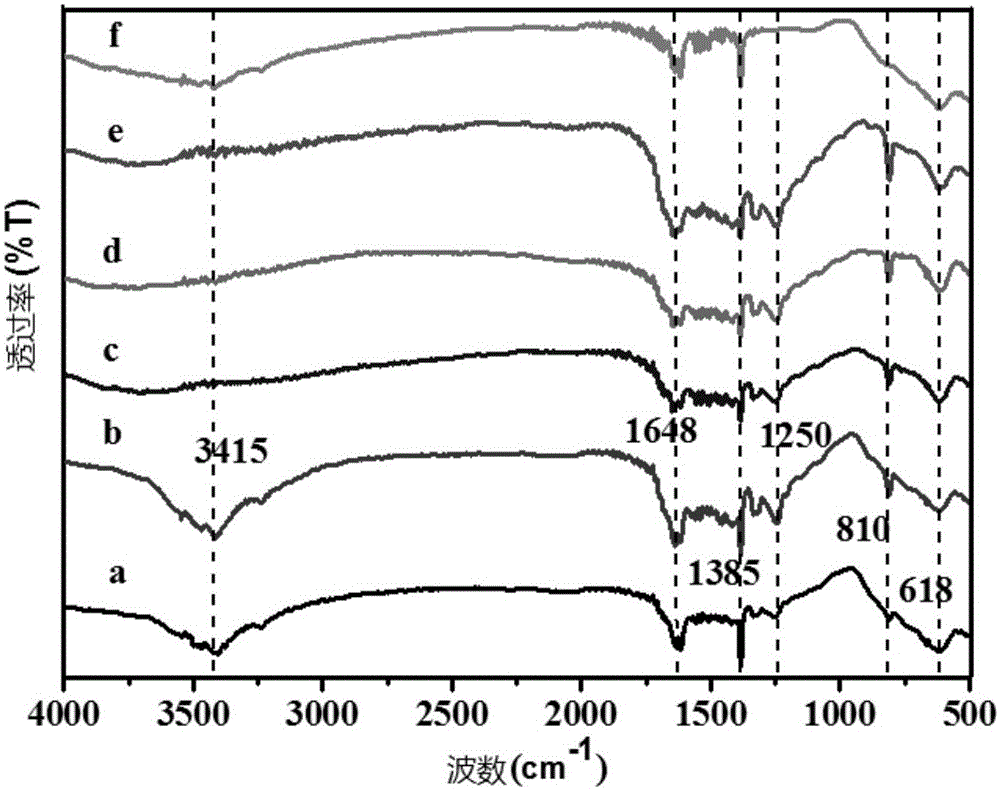
[0091] Infrared Spectrometry of Experimental Example 1 Sample
[0092] Infrared spectroscopy is used to measure that when a sample is irradiated by infrared light of continuously changing frequency, the molecule absorbs radiation of certain frequencies, and the change of dipole moment is caused by its vibration or bending motion, which causes the energy level to change from the ground state to the excited state. Transition, thus forming molecular absorption spectrum.
[0093] The samples used in this experimental example are the samples prepared in Example 1, Example 2, Example 3, Example 5, Comparative Example 1 and Comparative Example 2.
[0094] Operation method: Take the above catalyst sample, add sufficient amount of potassium bromide white powder, fully grind it with an agate mortar until it is evenly mixed, then move it into a mold, use a tablet press to manually press the catalyst into a transparent sheet, and measure it on an infrared spectrometer , set the paramet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com