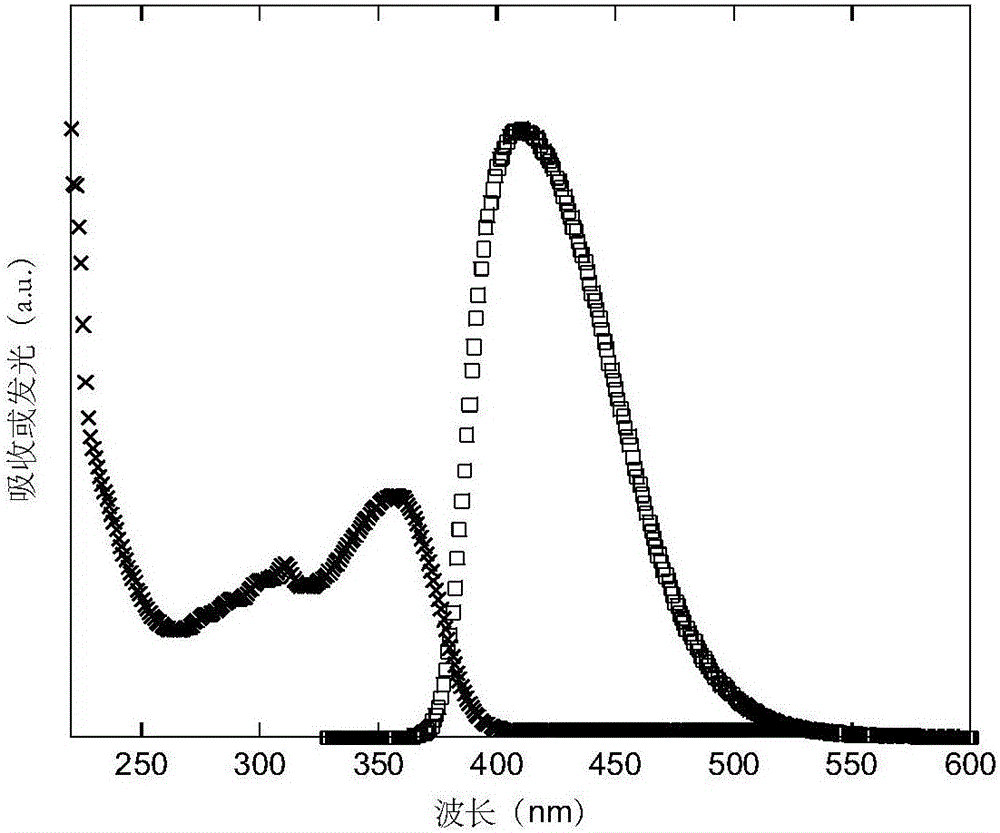
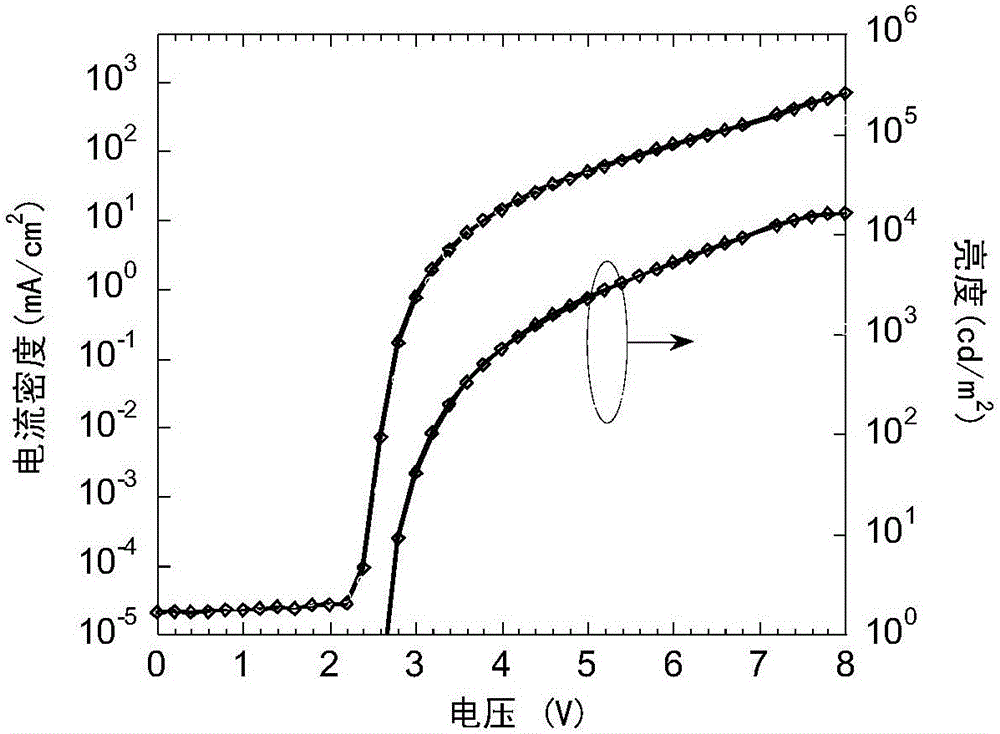
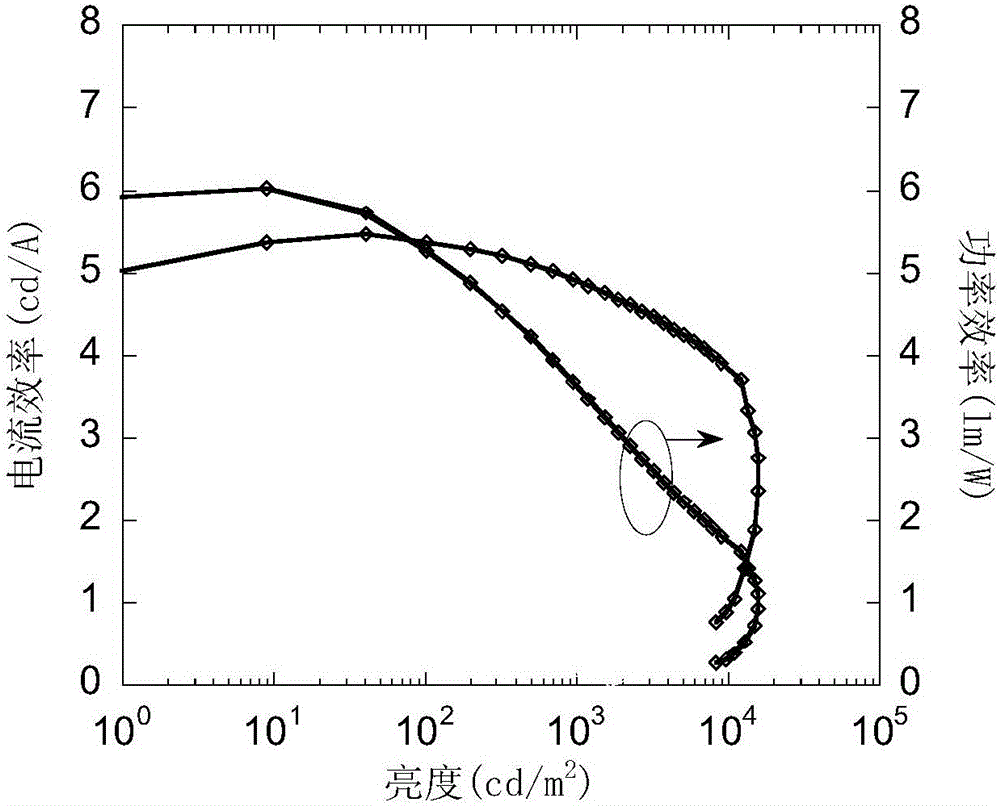
Spiro-di-thioxanthene-based small-molecule luminescent material and preparation and application thereof
A technology of light-emitting materials and thiaxanthene, which is applied in the fields of light-emitting materials, semiconductor/solid-state device manufacturing, electrical components, etc., to achieve the effects of good reproducibility, easy purification, and single material structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of a spiro-bisthiaxanthene-based small molecule luminescent material P1 in this example:
[0034] Synthesis of 2-bromodiphenylsulfide: o-bromoiodobenzene (50mmol, 14.3g), diphenyldisulfide (16.625mmol, 3.40g), Cu 2 S(1.194g), Fe(1.05g), K 2 CO 3 (8.625g) was dissolved in 125ml of DMSO, heated to 120°C overnight to obtain 2-bromodiphenylsulfide (3.84g), yield 93%. 1 HNMR: 7.55-7.59 (m, 1H), 7.36-7.48 (m, 5H), 7.13-7.18 (m, 1H), 7.01-7.07 (m, 1H), 6.92-6.96 (m, 1H). The reaction equation is as follows:
[0035]
[0036] For the synthesis of 2,7-dibromothioxanthone, refer to the publication specification of the invention patent application (CN200910048908.9), its structural formula and synthetic route are shown in the following formula, and the synthesis of intermediate M2, its structural formula and synthetic route are shown in the following formula:
[0037]
[0038] Preparation of the above compound 2: KOH (5.45 g, 97.3 mmol) and 60 ml of water wer...
Embodiment 2
[0044] Preparation of a spiro-bisthiaxanthene-based small molecule luminescent material P2 in this example:
[0045] The synthesis of P2, its structural formula and synthetic route are shown in the following formula:
[0046]
[0047]Dissolve 2.2gP1 in 30ML of dichloromethane, add 12ML of acetic acid and 3.0ml of 30% hydrogen peroxide, and react overnight at 80°C. After the reaction is complete, extract with dichloromethane, dry with anhydrous magnesium sulfate, and load the solid as a sample. Petroleum ether: dichloromethane = 2:1 through the column, 2.3g of white solid was isolated. Yield 88%. 1 HNMR: 8.22 (dd, J=8.1, 1.1 Hz, 1H), 7.54-7.46 (m, 1H), 7.42-7.34 (m, 1H), 7.04 (d, J=8.2 Hz, 1H).
Embodiment 3
[0049] Preparation of a spiro-bisthiaxanthene-based small molecule luminescent material P5 and P6 in this example:
[0050] Synthesis of intermediates M4, M5:
[0051]
[0052] The synthesis method of M4 is as in Example 1. Add compound 1 (2.65g, 100mmol) into a low-temperature reaction flask, dissolve it in 60mlTHF, and protect it with nitrogen gas. After sealing the device, add liquid nitrogen to cool to -78°C, and add dropwise within 30min. Butyllithium (1.6Minhexanes, 70mL, 110mmol) was incubated for 40min, then 2.21g of 3,7-dibromothioxanthone in THF was added in one go, and reacted overnight at room temperature. After the reaction is complete, THF is removed with a rotary evaporator, extracted with dichloromethane, and the product alcohol M3 is separated by a column. The product M3 was added with 20ml of acetic acid and an appropriate amount of hydrochloric acid, under nitrogen protection, stirred at 80°C overnight, and separated by column to obtain 0.83g of white so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com