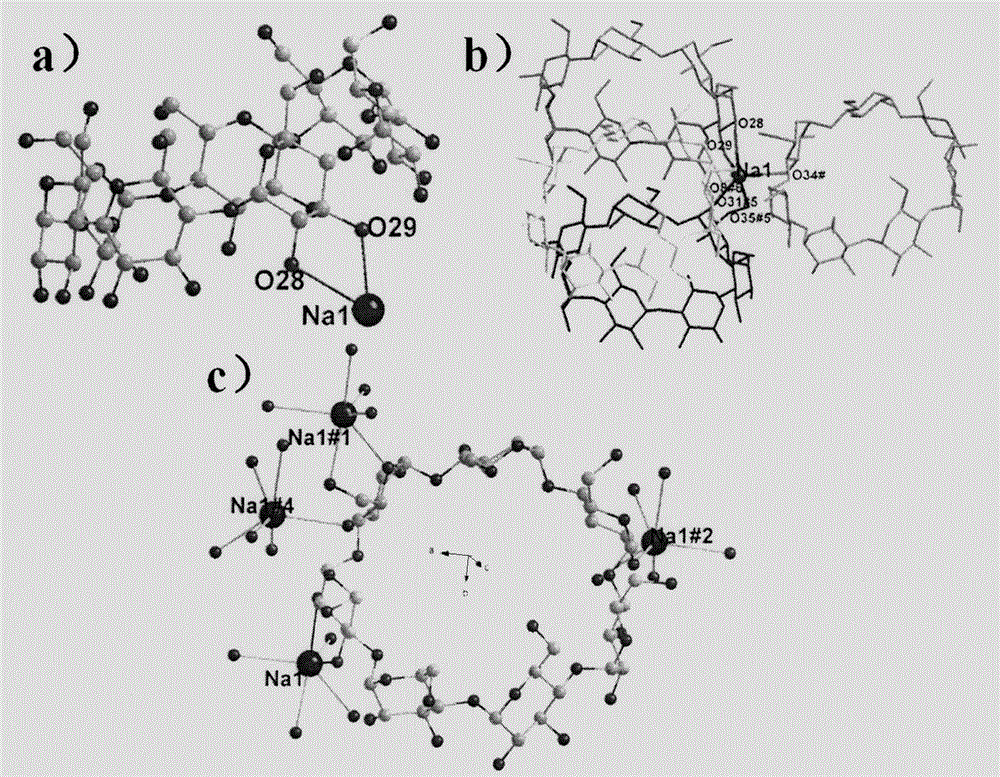
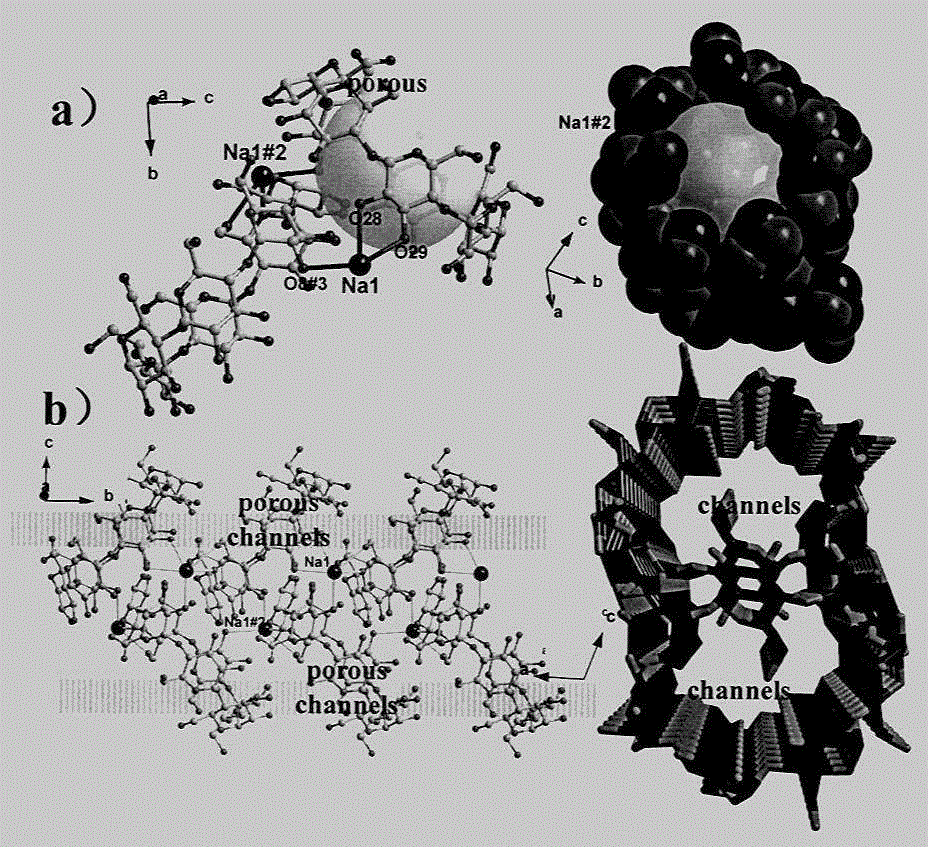
Cyclodextrin metal-organic framework compound and its preparation method and use
A metal-organic framework, cyclodextrin technology, applied in the direction of organic active ingredients, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., to achieve the effect of no toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The embodiment of the present invention also provides a preparation method of a cyclodextrin metal organic framework compound, comprising the following steps:
[0026] S1. Weigh a certain amount of β-CD and prepare its saturated solution with distilled water at 80-90°C, then cool to room temperature and filter to obtain β-CD crystals;
[0027] S2. Step S1 was repeated three times, and the obtained β-CD crystals were vacuum-dried at 100° C. to obtain β-cyclodextrin crystals;
[0028] S3. Weigh 1.135g (1mmol) of β-cyclodextrin and 0.448g (8mmol) of sodium hydroxide obtained in step S2 into 10mL water containing 40% (V 乙醇 :V 水 =6:4) in ethanol solution, stirred at room temperature for 30min to obtain a mixed solution;
[0029] S4. Transfer the mixed solution obtained in step S3 to a 15mL polytetrafluoroethylene low-pressure reactor, and perform a constant temperature reaction at 100° C. for 3 days to obtain a reactant;
[0030] S5. Cool the reaction product obtained in ...
Embodiment
[0037] Preparation of ASP-Na-CD-MOF inclusion compound
[0038] The clathrate of aspirin is prepared by the grinding method, the specific steps are as follows: Add anhydrous methanol to a certain amount of Na-CD-MOF and grind it into a paste at a specified temperature (30, 40, 50°C), then slowly add methanol Dissolved aspirin solution, triturated mixture for indicated times (1, 2, 3 h). The clathrate was washed three times with a small amount of anhydrous methanol, and freeze-dried to obtain a loose ASP-Na-CD-MOF clathrate powder, which was placed in a desiccator for future use. Prepare 3 copies in parallel.
[0039] According to the pre-experimental method, the inclusion rate of aspirin was used as the investigation index, and L 9 (3 4 ) was carried out in an orthogonal design experiment to investigate the influence of the feed ratio (A) of ASP and Na-CD-MOF, the inclusion temperature (B), the dropping time (C) and the inclusion time (D) of four factors, Choose three levels...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com