Preparation method of 2-amino-3,5-dibromobenzaldehyde
A technology of dibromobenzaldehyde and o-nitrobenzaldehyde, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of complex process and low yield, simplify the process and reduce the workload , to avoid the effect of condensation reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
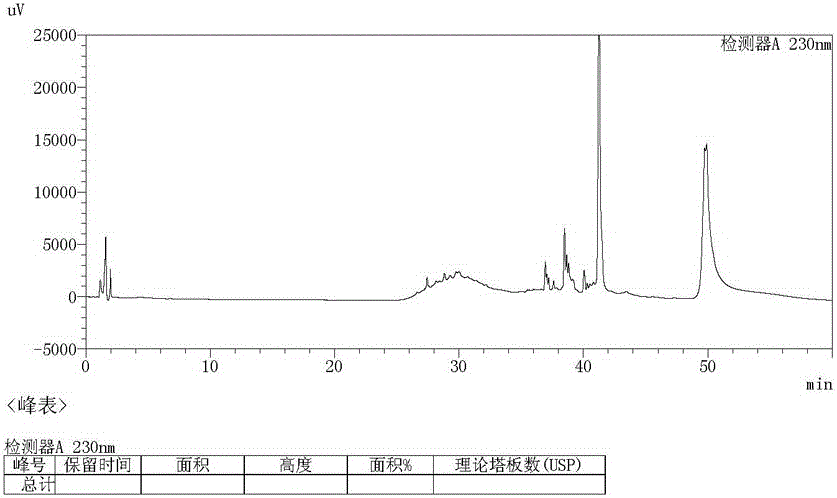
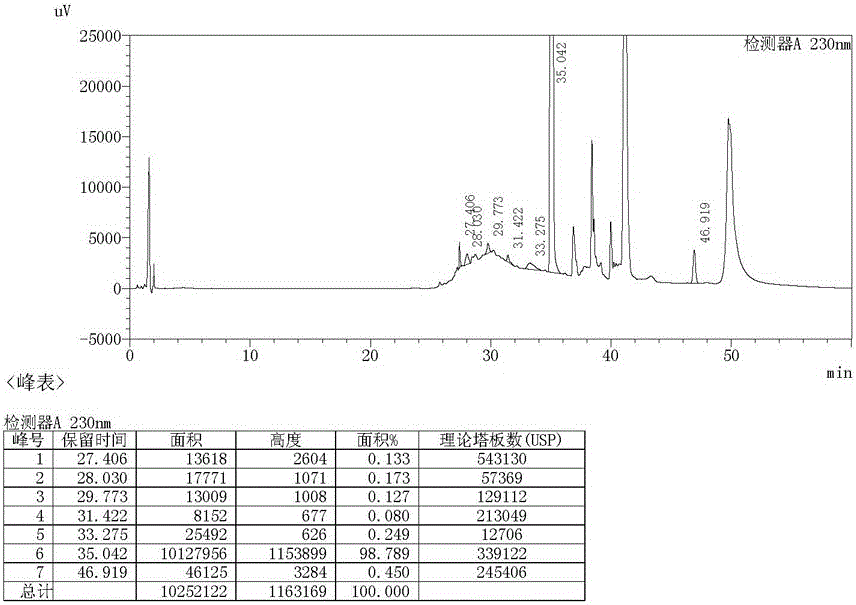
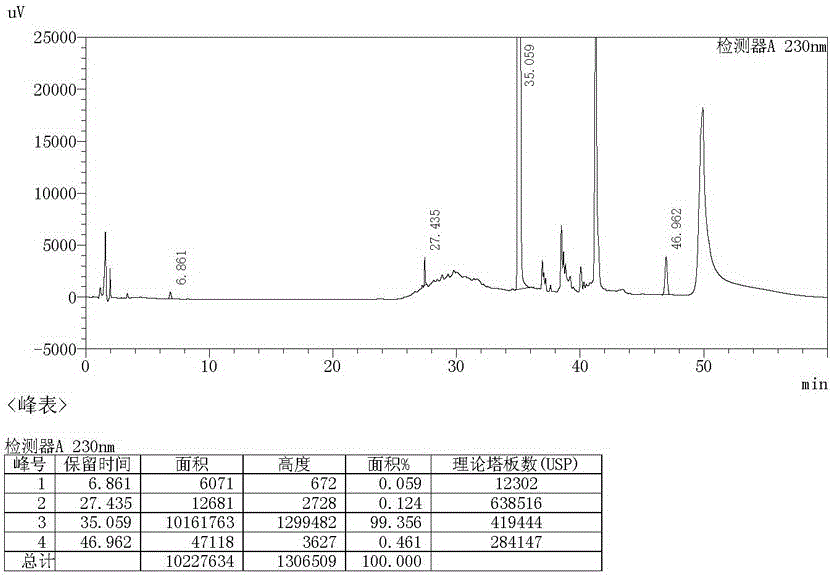
Image
Examples
Embodiment 1
[0019] Add 1.5g (10mmol) of o-nitrobenzaldehyde, 34mL of absolute ethanol, and 17mL of distilled water into a 100mL three-neck flask, and slowly heat up to 50°C under stirring. After the o-nitrobenzaldehyde is completely dissolved, add 34mL of acetic acid, 3.9g (70mmol) reduced iron powder and 3~4 drops of concentrated HCl, continue heating to 105°C; after reflux for 40min, stop the reaction.
[0020] The three-neck flask was cooled in a cold trap at 0°C, and after cooling, 3.1 g (20 mmol) of bromine was added dropwise, and reacted at room temperature for 120 min after the reduction was completed. Stop the reaction, filter with suction, wash with 10ml of water, then extract with 3*20ml of dichloromethane, then wash the dichloromethane layer with 2*20ml of saturated sodium bicarbonate solution and 2*20ml of water; dry with anhydrous sodium sulfate after washing The organic layer was rotary evaporated to obtain 2.76 g of a yellow solid.
[0021] Recrystallize with 10ml of aceto...
Embodiment 2
[0023] Add 1.5g (10mmol) of o-nitrobenzaldehyde, 40mL of absolute ethanol, and 10mL of distilled water into a 100mL three-neck flask, stir at room temperature, and then add 31mL of acetic acid and 2.2g (40mmol) of acetic acid to reduce Iron powder and 3~4 drops of concentrated HCl, continue to heat at 90°C; after refluxing for 60min, stop the reaction.
[0024] The three-neck flask was cooled in a cold trap at 0°C, and after cooling, 6.2g (40mmol) of bromine was added dropwise, and reacted at room temperature for 150min after the completion of the lowering. Stop the reaction, filter with suction, wash with 10ml of water, extract with 3*20ml of dichloromethane, wash the organic layer with 2*20ml of saturated sodium bicarbonate solution and 2*20ml of water; dry the organic layer with anhydrous sodium sulfate after washing , rotary evaporation to obtain a yellow solid 2.76g.
[0025] Recrystallize with 10ml of acetone to obtain 2.59g of light yellow solid, yield 92.8%, purity 99...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com