Application of melbine in preparing medicines for relieving cisplatin-induced acute kidney injury related diseases
A technology for acute kidney injury and metformin, which can be used in drug combinations, urinary system diseases, etc., and can solve problems such as metformin yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0028] Example 1 Effect of metformin on renal function in cisplatin-induced acute kidney injury.
[0029] Male CD1 mice with a body weight of 20-25 g were taken, 6-12 in each group, namely the equal volume normal saline group, the metformin alone group, the cisplatin model group and the metformin treatment group. in,
[0030] Equal volume of normal saline group: intraperitoneal injection, 10ml / kg, once a day, a total of 5 days;
[0031] Simple metformin group: intraperitoneal injection, 100mg / kg, once a day, 5 times in total;
[0032] Cisplatin model group: intraperitoneal injection, 20mg / kg, single administration;
[0033] Metformin treatment group: Metformin was administered three days in advance, intraperitoneal injection, 100mg / kg, once a day, followed by a single administration of cisplatin (intraperitoneal injection, 20mg / kg), metformin was treated for another two days, 48 hours after cisplatin injection , the eyeballs were picked to take blood, and the kidney tissu...
Embodiment 2
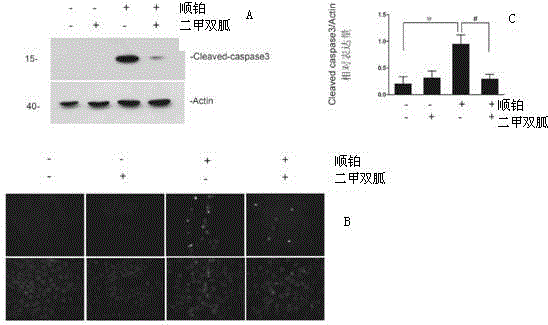
[0036] Example 2 Effect of metformin on cisplatin-induced tubular cell apoptosis.
[0037] TUNEL (TdT-mediateddUTPNick-EndLabeling) and cleaved-caspase3 positive renal tubular apoptotic cells were detected by tissue immunofluorescence.
[0038] The result is as figure 2 As shown, there were no obvious CleavedCaspase3 positive cells under the microscope in the normal saline group and the metformin group alone, there were 6.313±0.5995 positive cells in each high-power field in the cisplatin group, and there were 3.533±0.9366 positive cells in each high-power field in the metformin treatment group Positive cells (n=5). There were no obvious Tunel-positive cells under the microscope in the normal saline group and the metformin group alone, but there were 6.411±0.50 positive cells in each high-power field in the cisplatin group, and 3.35±0.6813 positive cells in each high-power field in the metformin treatment group (n= 5). It can be seen from the results that there are few apo...
Embodiment 3
[0039] Example 3 Effect of metformin on cisplatin-induced renal inflammatory cell infiltration.
[0040] The infiltration of inflammatory cells in the kidney was determined by immunofluorescence staining to detect the expression level of F4 / 80 (macrophage marker) and immunohistochemical staining to detect the expression level of Ly6b (neutrophil marker).
[0041] The result is as image 3 As shown, there were no obvious F4 / 80 positive cells under the microscope in the normal saline group and the metformin group alone. There were 5.483±0.3838 positive cells per high-power field in the cisplatin group, and 1.858±0.1425 positive cells in each high-power field in the metformin treatment group. Positive cells (n=5); there were no obvious Ly6B positive cells under the microscope in the normal saline group and the metformin group, there were 5.800±1.659 positive cells in each high-power field in the cisplatin group, and there were 1.433 positive cells in each high-power field in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com