Polypeptide inhibitor and application thereof
A technology of polypeptide inhibitors and uses, applied in the field of bioengineering, can solve the problems of poor treatment effect of human glioblastoma, achieve good enzyme activity inhibition effect, broaden the design, and improve the effect of mitophagy ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preliminary truncation screening of polypeptide inhibitors
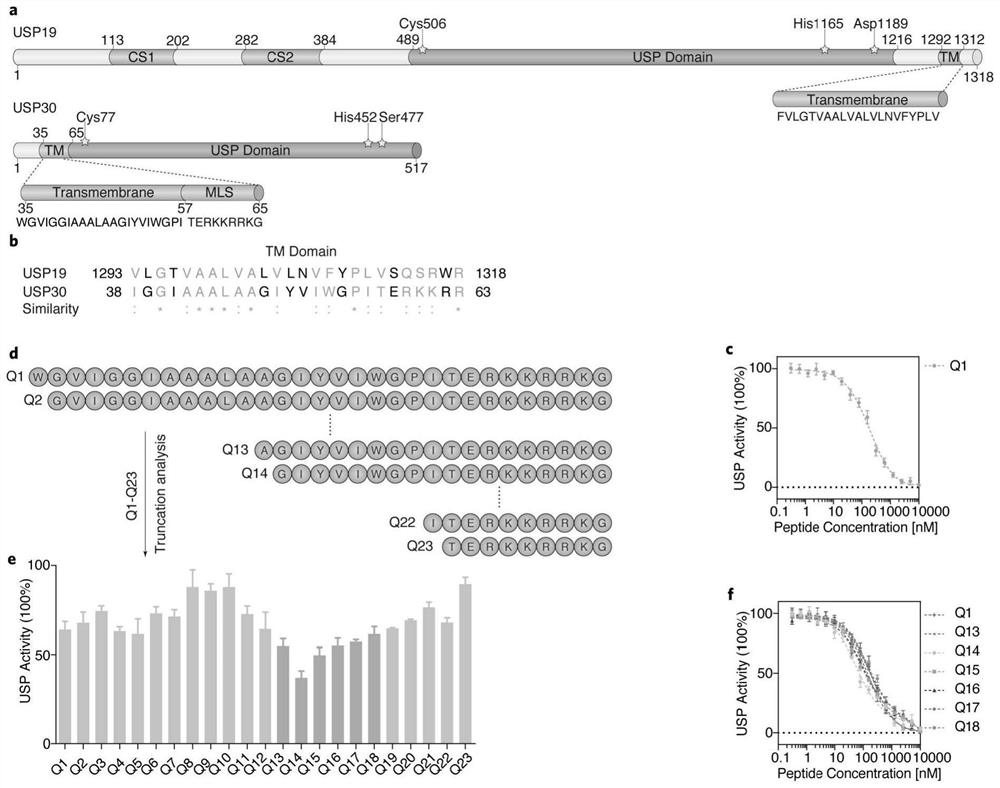
[0029] The present invention designs more effective USP30 polypeptide inhibitors based on the USP30 transmembrane domain. Such as figure 1 As shown, previous studies have shown (Lee, J.G., Kim, W., Gygi, S. & Ye, Y. Characterization of the deubiquitinating activity of USP19 and its role in endoplasmic reticulum-associated degradation. J Biol Chem 289, 3510-3517 ( 2014).) USP19 has a transmembrane domain of self-inhibitory activity. By comparing the sequences of the transmembrane domains of USP19 and USP30, we found that the transmembrane domains of the two have a high similarity ( figure 1 a, 1b). Therefore, we speculate that the transmembrane domain sequence of USP30 has a similar function of inhibiting autocatalytic activity.
[0030] The present invention synthesizes a polypeptide Q1 containing a transmembrane domain (TM) and a mitochondrial outer membrane localization sequence (MLS) based o...
Embodiment 2
[0034] Embodiment 2: the alanine screening of polypeptide inhibitor
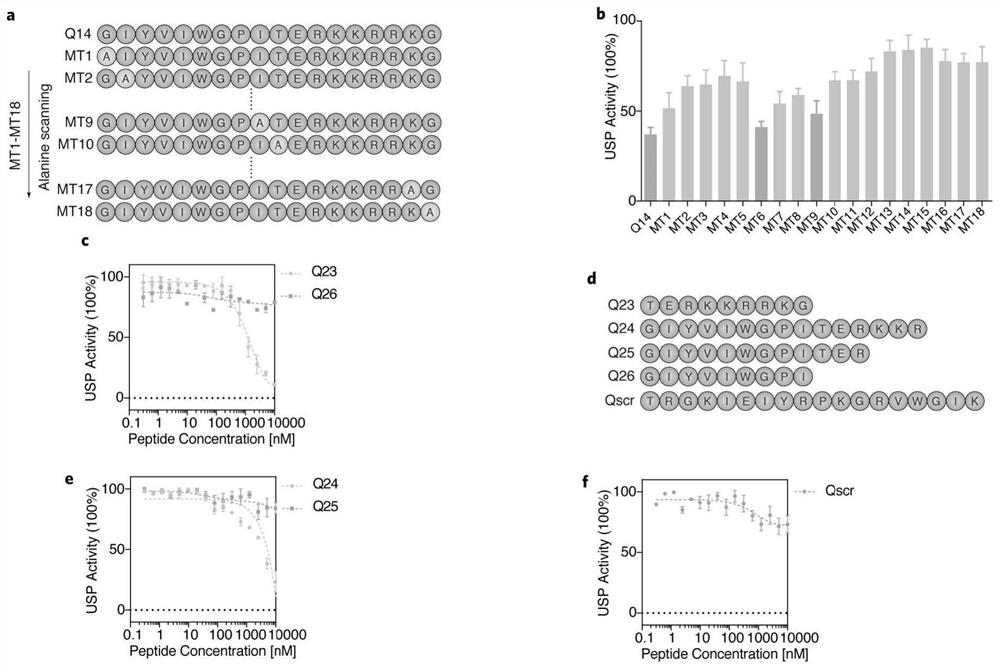
[0035] As shown above, the Q14 polypeptide in the transmembrane domain (TM) and mitochondrial outer membrane localization sequence (MLS) of USP30, which is rich in hydrophobic N-terminus and C-terminus rich in basic amino acids, can significantly inhibit the activity of USP30. The present invention intends to explore which residues on the polypeptide play an important role in inhibiting the function of USP30. Such as figure 2 As shown, the present invention has carried out alanine mutations to 18 amino acids of the polypeptide to detect the impact of amino acids on the inhibition of USP30 ( figure 2 a). From the results of enzyme activity, it can be seen that the mutation of the amino acid on the Q14 polypeptide has a greater impact on the catalytic activity of Q14 to inhibit the USP30 protein, and the mutation of the N-terminal of the polypeptide has a smaller impact than the C-terminal, especially MT6 ...
Embodiment 3
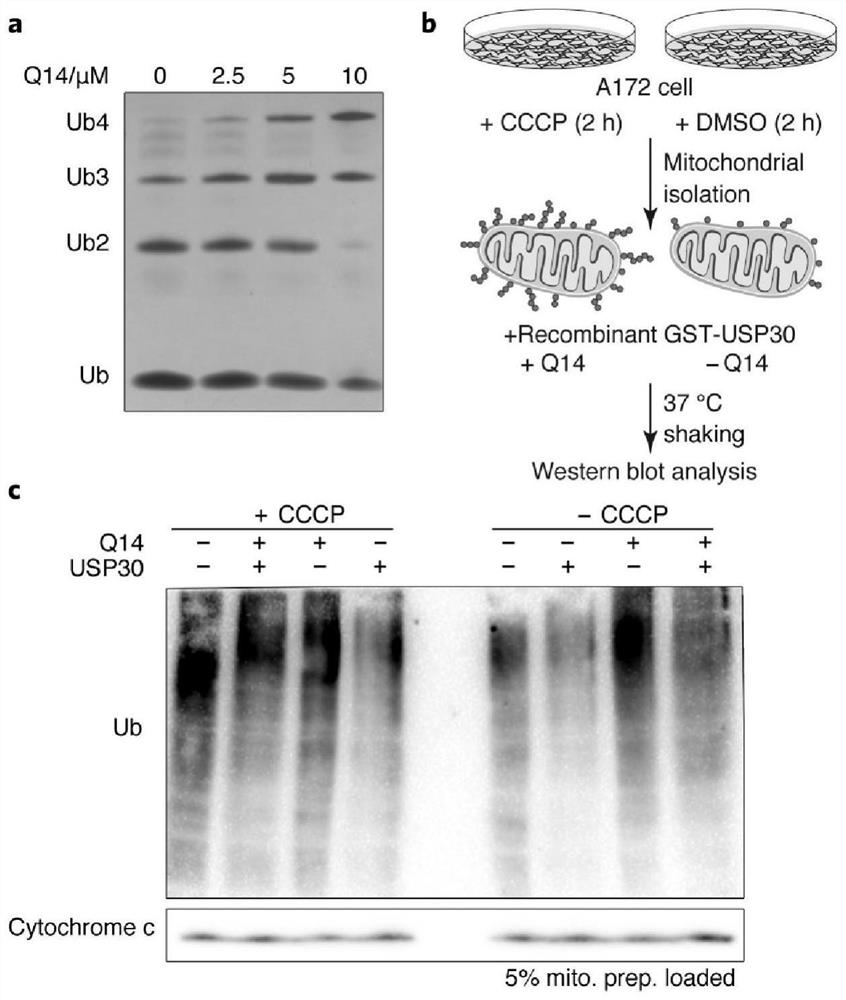
[0037] Previous studies have shown that USP30 protein is a special USP-type deubiquitinase, which is more inclined to catalyze the hydrolysis of ubiquitin chains with a compact structure, and the hydrolysis speed of Lys 6-type ubiquitin chains is the fastest. Therefore, the present invention pre-incubated Q14 polypeptides with different concentrations and 2 μg USP30 protein for 30 minutes, then added the tetraubiquitin chain of Lys 6, stopped the reaction after 15 minutes in a 37-degree water bath, and detected the hydrolyzed protein by silver staining experiment. The ubiquitin chain was used to evaluate the inhibitory effect of polypeptide Q14 on the catalytic activity of USP30. Consistent with the results of the Ub-AMC enzyme digestion assay, with the increase of Q14 concentration, compared with the control, the monoubiquitin chains hydrolyzed gradually decreased ( image 3 a). This indicated that Q14 gradually inhibited the catalytic activity of USP30 with increasing conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com