Pharmaceutical lansoprazole composite granules for treating digestive system diseases
A technology for digestive system diseases and lansoprazole, applied in the field of medicine, can solve problems such as affecting drug quality and unsatisfactory results, and achieve the effects of good stability, excellent fluidity, and improved dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
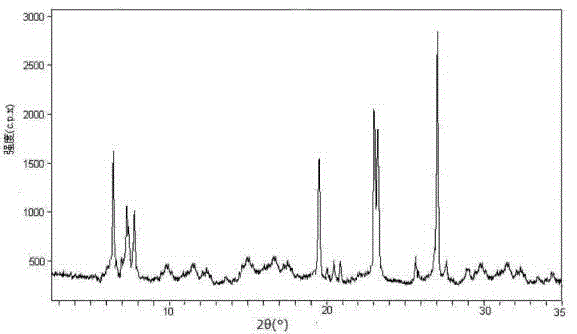
[0042] Example 1 Preparation of Lansoprazole Crystals
[0043] (1) Take the raw material of lansoprazole and add it to methanol. The volume of methanol is 5 times the mass of lansoprazole, and the temperature is raised to 35°C
[0044] (2) Stir until all dissolved;
[0045] (3) Add activated carbon to decolorize and filter to obtain a clear solution;
[0046] (4) Transfer the clear solution into a pressure vessel, and under the condition of controlling the pressure in the pressure vessel at 2.0Mpa and stirring, add dropwise a mixed solvent of ethyl acetate and ether at 5°C. The stirring speed is controlled at 35rpm, ethyl acetate , The volume consumption of ether is 5 times the volume of deionized water, and the volume ratio of ethyl acetate and ether is 3:1;
[0047] (5) After dripping, release the pressure, cool the solution to -5°C at a rate of 5°C / min, let it stand for 3 hours, filter, wash, and dry under reduced pressure to obtain lansoprazole crystals.
[0048] The prepared lanso...
Embodiment 2
[0049] Example 2 Preparation of Lansoprazole Granules
[0050] Prescription: In parts by weight, 0.15 parts of lansoprazole crystal compound prepared in Example 1, 2.4 parts of sucrose, 0.5 parts of anhydrous sodium carbonate, 0.4 parts of croscarmellose sodium, nordihydrogen 0.04 parts of lignoic acid, 0.7 parts of 95% ethanol, 0.02 parts of talc.
[0051] Preparation:
[0052] 1) Raw and auxiliary materials processing: use a vibrating sieve powder machine to pass the raw material lansoprazole through an 80-mesh sieve, and use a pulverizer to crush anhydrous sodium carbonate and pass a 120-mesh sieve;
[0053] 2) Weigh according to the technological prescription;
[0054] 3) Mixing granulation: Add the prescription amount of lansoprazole, sucrose, anhydrous sodium carbonate, croscarmellose sodium, and nordihydroguaiaretic acid to the wet mixing granulator, turn on Stir the motor for dry mixing for 10 minutes, add the prescription amount of 95% ethanol, wet-mix for 90-120 seconds to ...
Embodiment 3
[0058] Example 3 Preparation of Lansoprazole Granules
[0059] Prescription: In parts by weight, 0.15 parts of lansoprazole crystal compound prepared in Example 1, 2.6 parts of sucrose, 0.6 parts of anhydrous sodium carbonate, 0.5 parts of croscarmellose sodium, nordihydrogen 0.05 parts of lignoic acid, 0.8 parts of 95% ethanol, 0.03 parts of talc.
[0060] Preparation:
[0061] 1) Raw and auxiliary materials processing: use a vibrating sieve powder machine to pass the raw material lansoprazole through an 80-mesh sieve, and use a pulverizer to crush anhydrous sodium carbonate and pass a 120-mesh sieve;
[0062] 2) Weigh according to the technological prescription;
[0063] 3) Mixing granulation: Add the prescription amount of lansoprazole, sucrose, anhydrous sodium carbonate, croscarmellose sodium, and nordihydroguaiaretic acid to the wet mixing granulator, turn on Stir the motor for dry mixing for 10 minutes, add the prescription amount of 95% ethanol, wet-mix for 90-120 seconds to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com