Pharmaceutical lansoprazole compound for treating gastric ulcer
A lansoprazole and compound technology, applied in the directions of drug combination, digestive system, organic chemistry, etc., can solve problems such as unsatisfactory results and influence drug quality, and achieve the effects of small content change, improved dissolution rate, and good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
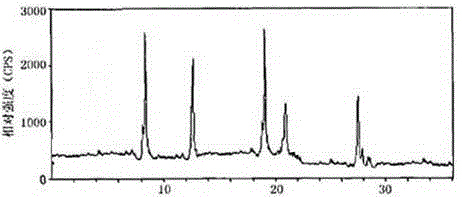
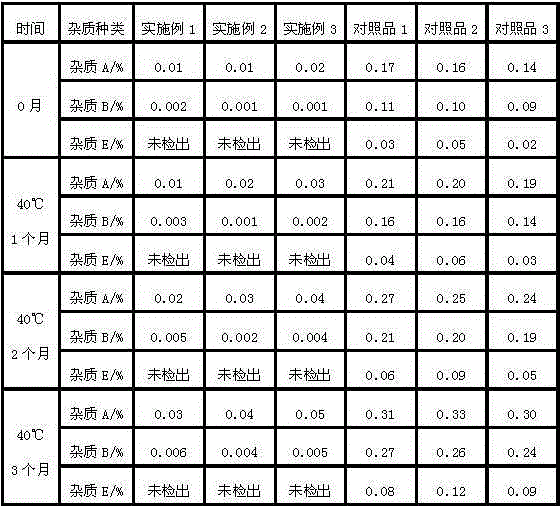
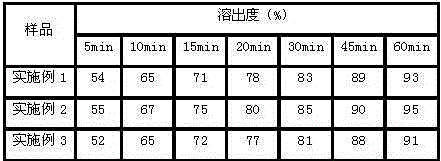
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of lansoprazole crystal compound
[0035] (1) The crude product of lansoprazole is ground, passed through a 80 mesh sieve, and then added to a mixed solution of methanol, isopropanol and methyl furan whose volume is 5 times the weight of lansoprazole, methanol, isopropanol and formaldehyde The volume ratio of base furan is 5:3.5:2, stirs 15 minutes with the speed of 120 revs / min;
[0036] (2) Add a mixed solution of tetrahydrofuran and water whose volume is 8 times the weight of lansoprazole under stirring at a speed of 50 rpm, and the volume ratio of tetrahydrofuran:water is 1:1, and the temperature is raised to 20°C at the same time;
[0037] (3) After the solution has been added, leave it to stand for 2 hours, add dropwise the mixed solution of dichloromethane and acetone whose volume is 8 times of the weight of lansoprazole under the condition of 80 rev / min speed stirring, the mixture of dichloromethane and acetone The volume ratio is 1:1.5...
Embodiment 2
[0040] Example 2: Preparation of lansoprazole crystal compound
[0041] (1) The crude product of lansoprazole is ground, passed through a 100-mesh sieve, and then added to a mixed solution of methanol, isopropanol and methyl furan whose volume is 6 times the weight of lansoprazole, methanol, isopropanol and formaldehyde The volume ratio of base furan is 5:3.5:2, stirs 20 minutes with the speed of 150 rev / mins;
[0042] (2) Add a mixed solution of tetrahydrofuran and water whose volume is 10 times the weight of lansoprazole under stirring at a speed of 60 rpm, and the volume ratio of tetrahydrofuran:water is 1:1, and the temperature is raised to 27.5°C at the same time;
[0043] (3) After the solution has been added, leave it to stand for 2.5 hours, add dropwise the mixed solution of dichloromethane and acetone whose volume is 9 times of the weight of lansoprazole under the condition of 90 rev / min speed stirring, the mixture of dichloromethane and acetone The volume ratio is 1...
Embodiment 3
[0046] Example 3: Preparation of lansoprazole crystal compound
[0047] (1) The crude product of lansoprazole is ground, passed through a 120 mesh sieve, and then added to a mixed solution of methanol, isopropanol and methyl furan whose volume is 7 times the weight of lansoprazole, methanol, isopropanol and formaldehyde The volume ratio of base furan is 5:3.5:2, stirs 25 minutes with the speed of 180 rev / mins;
[0048] (2) Add a mixed solution of tetrahydrofuran and water whose volume is 12 times the weight of lansoprazole under stirring at a speed of 70 rpm, and the volume ratio of tetrahydrofuran:water is 1:1, and the temperature is raised to 35°C at the same time;
[0049] (3) After the solution has been added, leave it to stand for 2-3 hours, add dropwise the mixed solution of dichloromethane and acetone whose volume is 10 times the weight of lansoprazole with 100 rev / min speed stirring, dichloromethane and The volume ratio of acetone is 1:1.5, and the uniform dropwise ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com