Hepatocyte serum-free culture medium
A technology of serum-free medium and hepatocytes, which is applied in the field of animal cell culture, can solve the problems of the influence of experimental results, irreversible damage of cells, and easy carrying of prions in serum.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Concentration screening of each component of the hepatocyte serum-free medium of the present invention
[0031] 1. Orthogonal optimization design screens the concentration of each component in the medium:
[0032] Orthogonal test scheme: This study includes 4 factors including sericin (A), dexamethasone (B), hepatocyte growth factor (HGF (C)) and epidermal growth factor (EGF (D)). 3 levels (see Table 1), so choose L9 (3 4 ) orthogonal table, the test scheme is shown in Table 2. The study selected DEME high-glucose type as the basal medium, added insulin 5μg / ml, transferrin 5μg / ml, sodium selenite 10μg / L, linoleic acid 11ng / ml, HEPES10mmol / L, penicillin and streptomycin 100U / ml , and according to the experimental scheme in Table 2, the corresponding doses of 4 components were added, and mixed to prepare 9 groups of culture media.
[0033] Table 1 Orthogonal design factor level table
[0034]
[0035] Table 2 Orthogonal test scheme table
[0036]
[0037] 2. In...
Embodiment 2
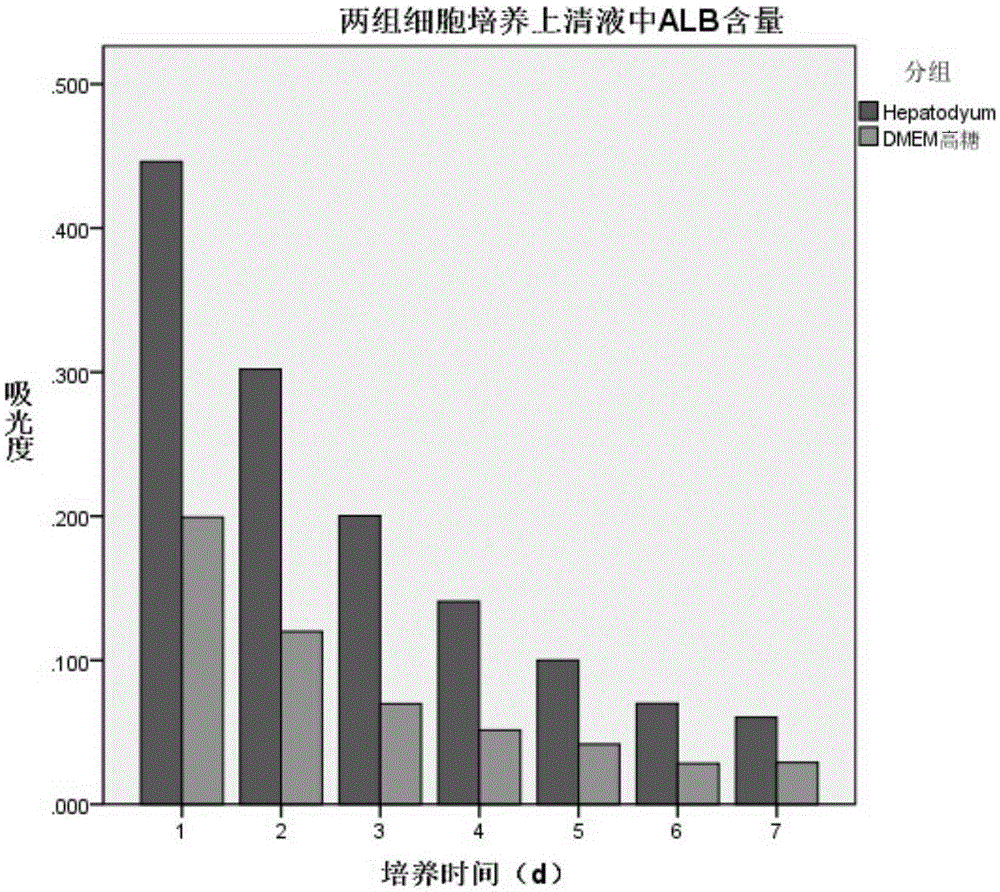
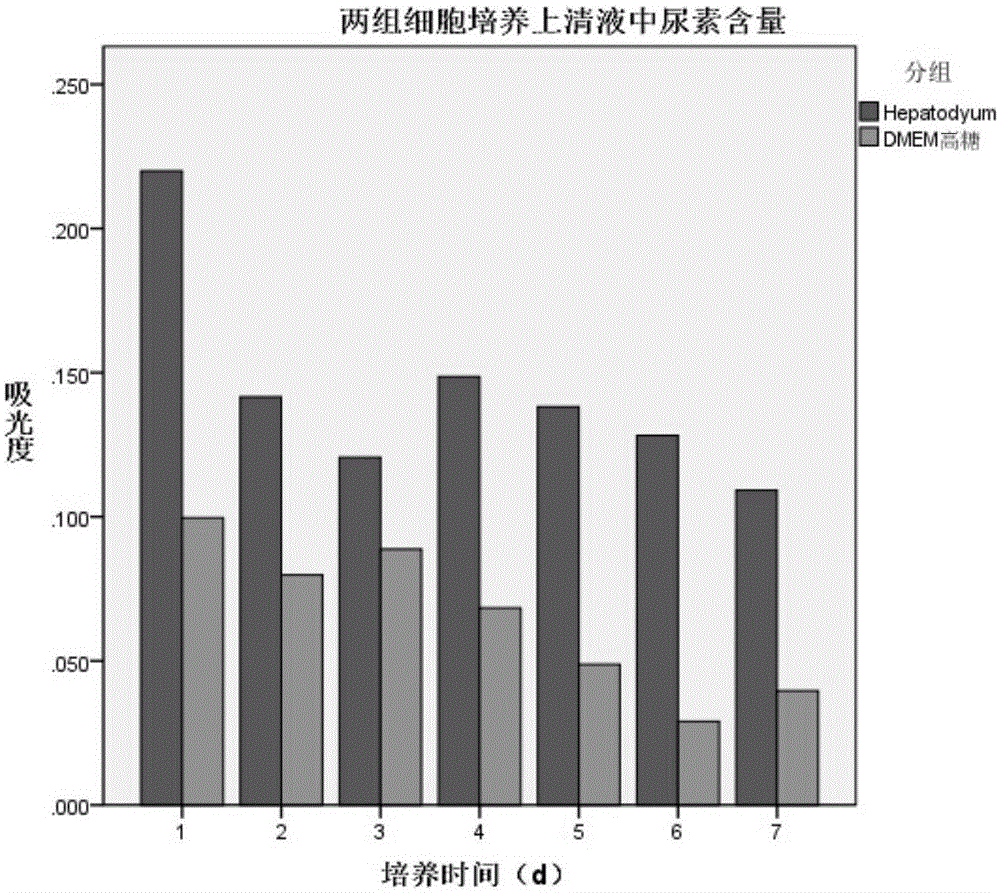
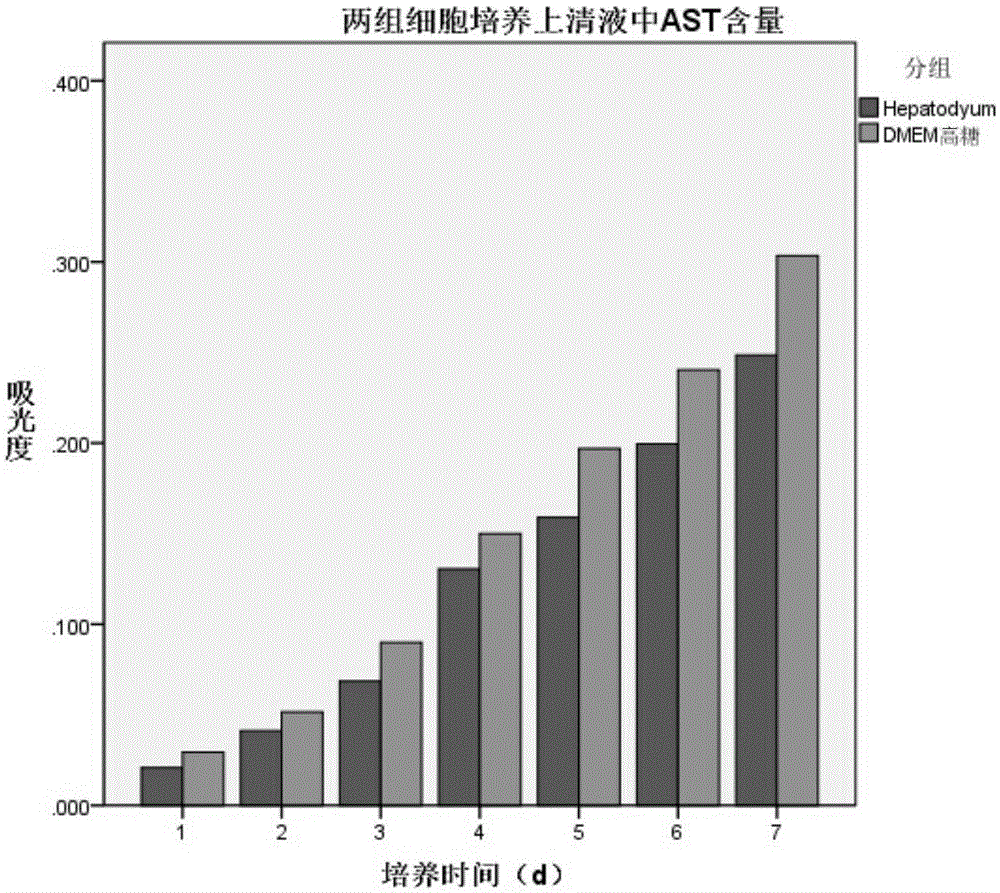
[0039] Hepatocyte serum-free culture medium of the present invention is to the culture effect of hepatocyte
[0040] 1. Detection methods of various indicators
[0041] (1) Method for detecting cell viability: take logarithmic phase cells and make 2×10 4 / ml cell suspension, seeded in 96-well plate (100μl / well), in 5% CO 2 and cultured at 37°C for 24 hours, take 5 multiple wells every day, add 10 μl CCK-8 reagent to each well, incubate in the above environment for 1 hour, measure the absorbance at 450nm wavelength with a microplate reader, and draw the absorbance-time curve , take the maximum value of the absorbance of each group.
[0042] (2) Method for cell function detection: Inoculate 1×10 cells per well in a 6-well plate 5 cells, and add 3ml medium, replace the medium every 24h, in 5% CO 2 and 37°C environment, the cell culture supernatant was collected every day, the albumin content was measured by ELISA method, and the AST, LDH and urea content were measured by micr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com