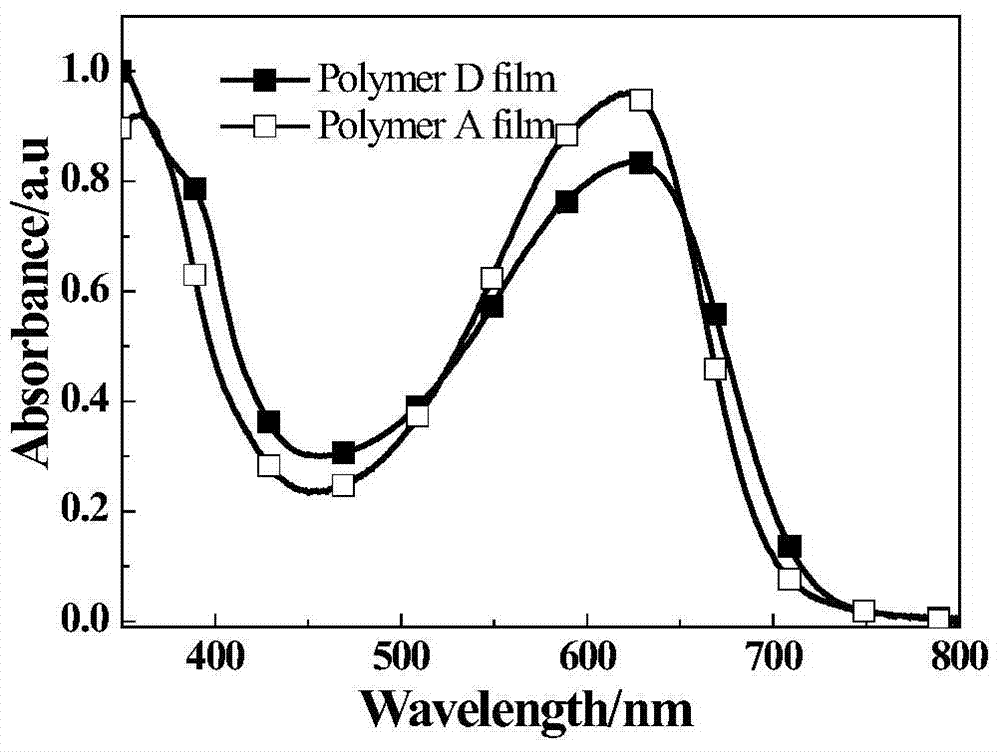
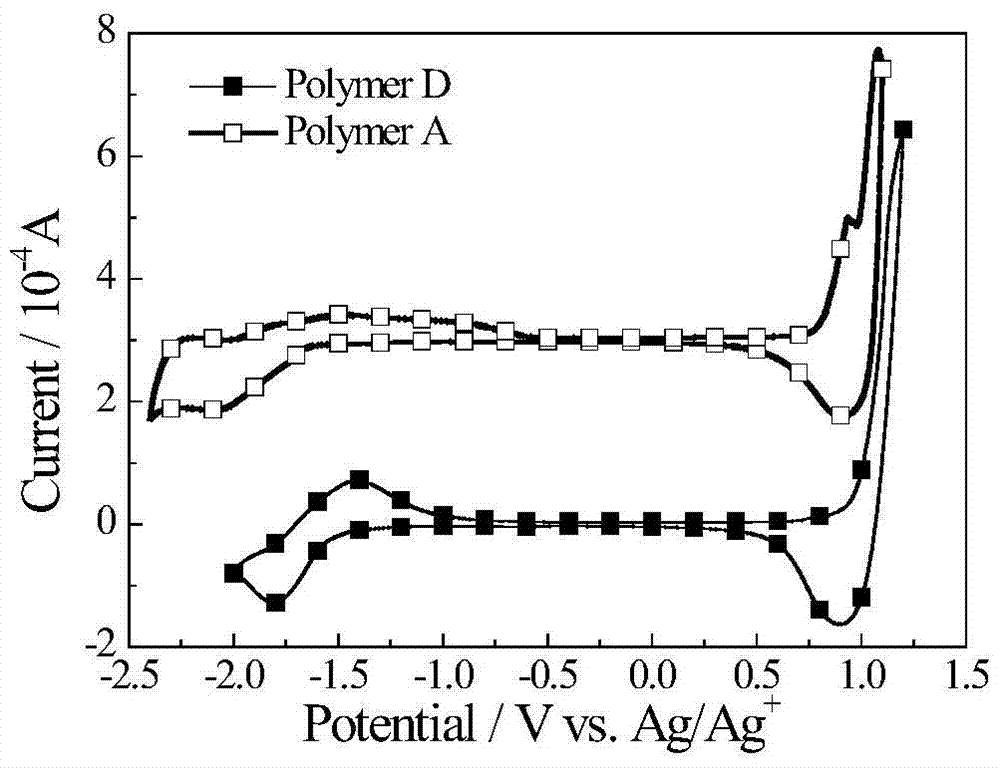
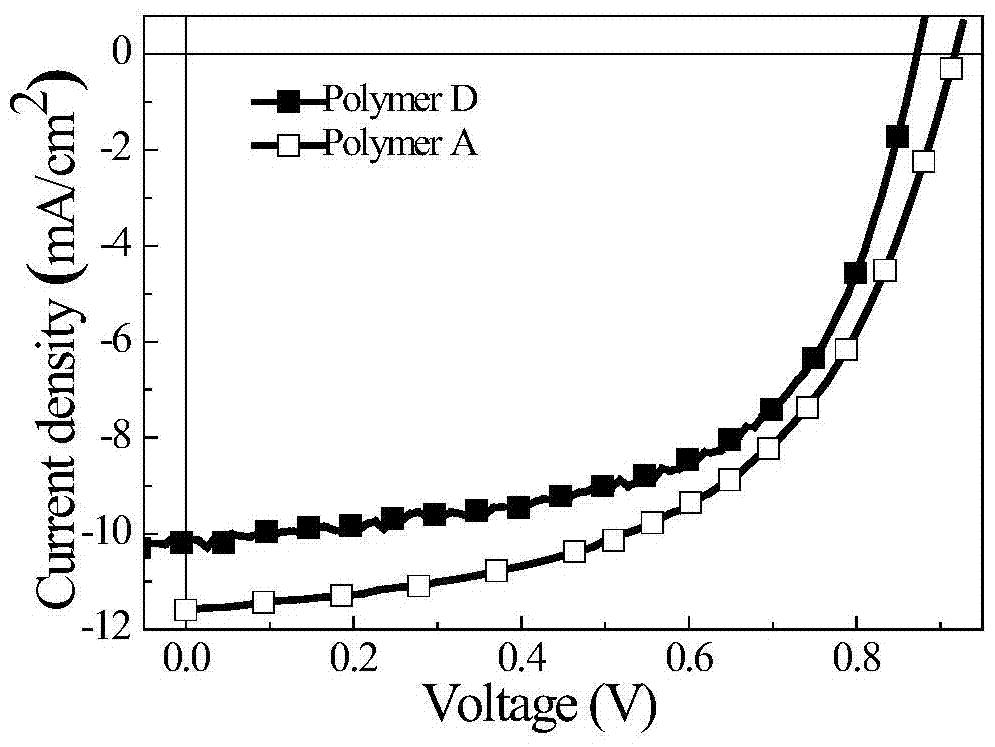
Conjugated polymers of fluorine-containing phenanthrenequinoxaline and thiophene
A technology of conjugated polymer and substituted quinoxaline, applied in the field of functional polymers, can solve the problems of reduced degree of conjugation of polymer molecules, large optical energy gap that cannot effectively absorb sunlight, and hindering the improvement of photoelectric conversion efficiency of devices. , to reduce the optical energy gap and improve the photovoltaic performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the synthesis of intermediate
[0038] (1) Preparation of 4-fluoro-3,6-dibromo-1,2-phenylenediamine (compound 1)
[0039] 5-Fluoro-4,7-dibromo-2,1,3-benzothiadiazole (5g, 0.016mol) was dissolved in 150ml of absolute ethanol, and NaBH4 (11.1g, 0.29mol) was added in portions at 0°C , and then reacted at room temperature for 20h. After the reaction, ethanol was removed by vacuum distillation, 160ml of water was added, extracted with ethyl acetate, the organic phase was washed with saturated brine, and finally dried with anhydrous MgSO4. Concentrate to remove the organic solvent, and the obtained crude product is chromatographed on a silica gel column with n-hexane / ethyl acetate (25:1 by volume) to obtain 3.5 g of 4-fluoro-3,6-dibromo-1,2-phenylenediamine , yield 78%.
[0040] (2) Preparation of 1,2-bis(3-octyloxyphenyl)ethanedione (compound 2)
[0041] Add LiBr (5.25g, 60.4mmol) to the THF solution (40ml) of CuBr (4.33g, 30.2mmol), stir and dissolve at ro...
Embodiment 2
[0047] Example 2: Polymer A
[0048]
[0049] The reaction was carried out under the protection of nitrogen, 11-fluoro-10,13-dibromo-2,7-dioctyloxybenzodiazepine (213.7mg, 0.3mmol) and 2,5-bis(trimethyltin) Thiophene (198.6mg, 0.3mmol) was dissolved in 10ml of toluene, nitrogen gas was passed for 0.5h, catalyst tris(dibenzylideneacetone)dipalladium (5.5mg) and ligand tri-o-cresylphosphine (9.8mg) were added, and continued After aeration for 0.5h, heating was started, and the reaction was refluxed for 24h. The system was naturally cooled at room temperature, added dropwise to methanol to settle, filtered, and the collected polymer was dried in a vacuum oven at 50°C for 12h. The obtained polymer was sequentially washed with methanol, n-hexane, Chloroform Soxhlet extraction, concentrated chloroform extract, again dropped into methanol for sedimentation, to obtain 175 mg of polymer A as a purple-black fibrous solid with a yield of 88%, a number average molecular weight of 192.3...
Embodiment 3
[0052] Example 3: Polymer B
[0053]
[0054] Same as in Example 2, changing the deficient unit to 11-fluoro-10,13-dibromo-3,6-dioctyloxybenzodiazepine, using exactly the same polymerization method to obtain polymer B with a yield of 76 %, the number average molecular weight is 153.67kDa, and the distribution coefficient is 2.75.
[0055] Electrochemical test: use CHI660D electrochemical workstation, use glassy carbon electrode as working electrode, platinum wire electrode as counter electrode, Ag / Ag+ electrode as reference electrode, Bu4N·PF6 as electrolyte, in acetonitrile solvent, through cyclic voltammetry The HOMO energy of the polymer B thin film was determined to be -5.58eV.
[0056] Photovoltaic performance research: Using the sandwich cell structure of ITO / PEDOT:PSS / polymer:PC71BM / LiF / Al, the polymer B and the acceptor material PC71BM are made into a photovoltaic cell according to a certain weight ratio, with an effective area of 0.16cm2, in Newport Thermal Ori...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com