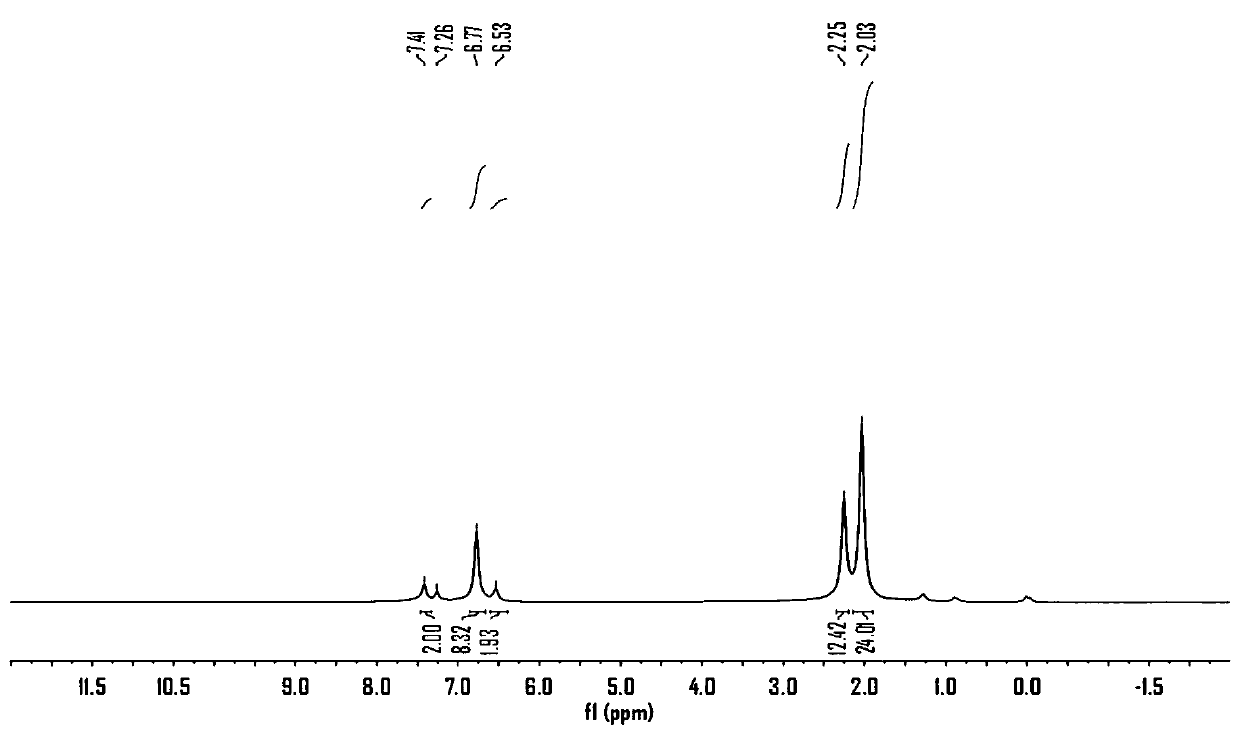
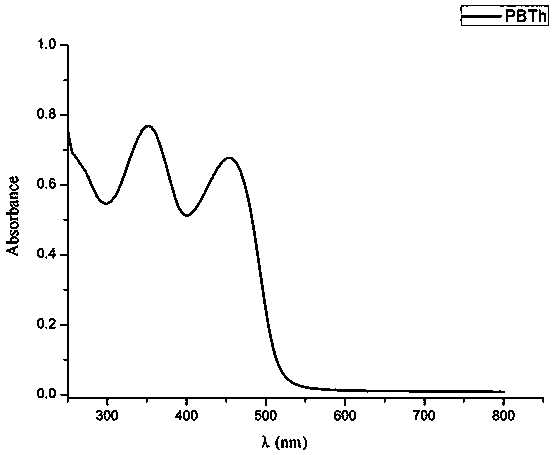
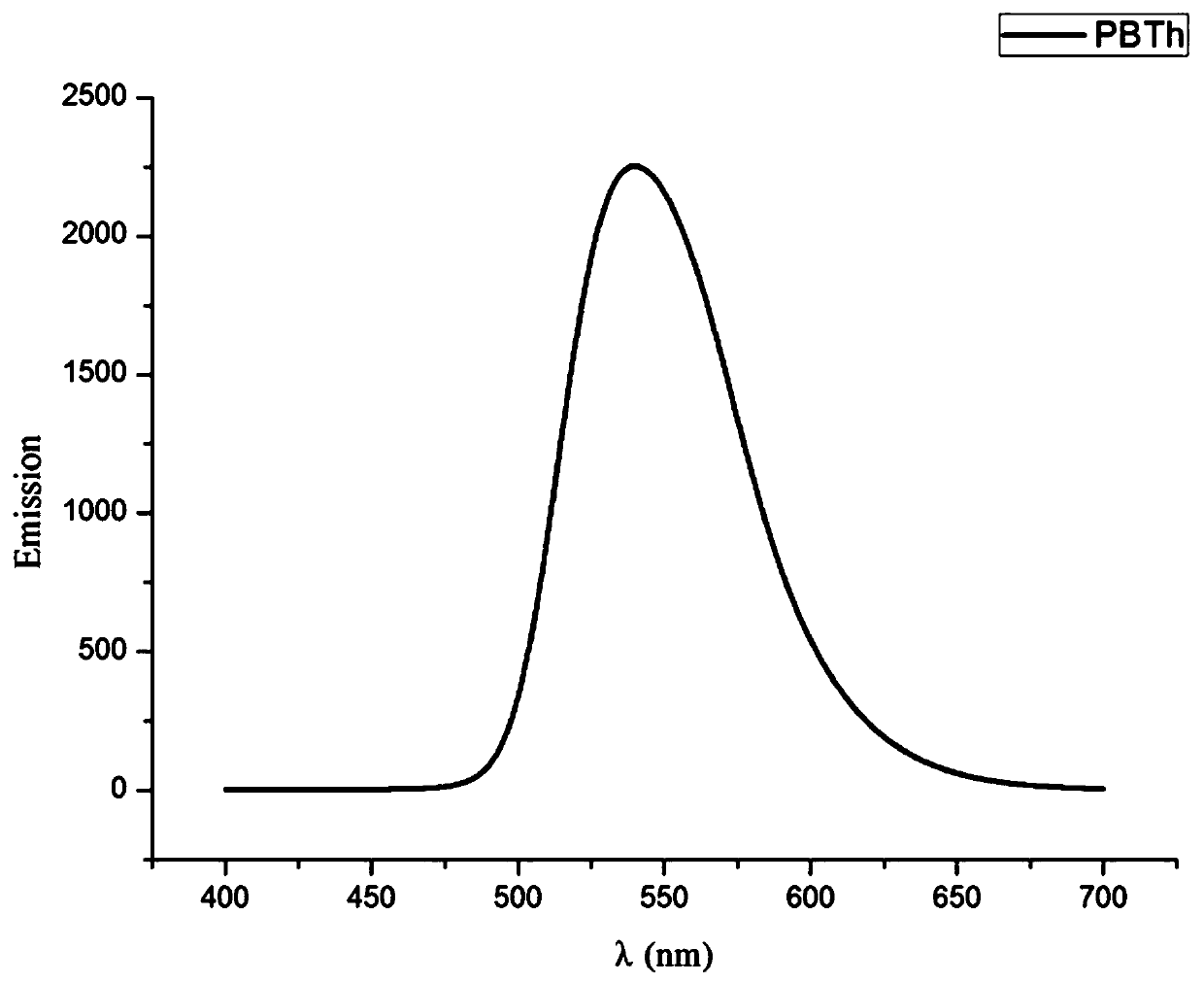
Thiophene-containing conjugate organo-boron polymer and preparation method therefor
A technology of organic boron and polymers, applied in the field of functional organic semiconductor materials, can solve the problems of reducing application value, and achieve the effects of cheap medicines, short production cycles, and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A preparation method of a thiophene-containing conjugated organic boron polymer, comprising the following steps:
[0036] (1) Synthesis of raw material A, 1,4-bis(trimethylsilylacetylene)-2,5-dibromobenzene
[0037] Under anaerobic conditions, weigh 30 mmol of 1,4-dibromo-2,5-diiodobenzene, 1.5 mmol of bis(triphenylphosphine)palladium chloride, and 3 mmol of cuprous iodide in 150 In a mixed solution of degassed toluene and 75 mL of degassed diisopropylamine, 63 mmol of trimethylsilylacetylene was added to the above reaction solution, and stirred for 24 hours. After the reaction was complete, quenched by adding 150 mL of water, extracted with dichloromethane (3 x 50 mL), combined organic phase, Mg 2 SO 4 Dry, spin to dry the solvent, and purify by column chromatography (n-hexane) to give a light yellow solid (yield: 25.5 mmol, 85%), 1 H NMR (400 MHz, CDCl 3 ): δ7.67 (s, 2H), 0.27 (s,18H).
[0038] (2) Synthesis of raw material B, 1,4-bis(bis(trimethylphenylboron))-2...
Embodiment 2
[0052] A preparation method of a thiophene-containing conjugated organic boron polymer, comprising the following steps:
[0053] (1) Synthesis of raw material A, 1,4-bis(trimethylsilylacetylene)-2,5-dibromobenzene
[0054] Under anaerobic conditions, weigh 40 mmol of 1,4-dibromo-2,5-diiodobenzene, 2 mmol of bis(triphenylphosphine)palladium chloride, and 4 mmol of cuprous iodide in 200 In a mixed solution of mL degassed toluene and 100 mL degassed diisopropylamine, 84 mmol of trimethylsilylacetylene was added to the above reaction solution, and stirred for 24 h. After the reaction was complete, quenched by adding 200 mL of water, extracted with dichloromethane (3 x 70 mL), combined organic phase, Mg 2 SO 4 Dry, spin to dry the solvent, and purify by column chromatography (n-hexane) to give a pale yellow solid (yield: 33.2 mmol, 83%), 1 H NMR (400 MHz, CDCl 3 ): δ7.67(s, 2H), 0.27(s, 18H).
[0055] (2) Synthesis of raw material B, 1,4-bis(bis(trimethylphenylboron))-2,5-bis(...
Embodiment 3
[0064] A preparation method of a thiophene-containing conjugated organic boron polymer, comprising the following steps:
[0065] (1) Synthesis of raw material A, 1,4-bis(trimethylsilylacetylene)-2,5-dibromobenzene
[0066] Under anaerobic conditions, weigh 50 mmol of 1,4-dibromo-2,5-diiodobenzene, 2.5 mmol of bis(triphenylphosphine)palladium chloride, and 5 mmol of cuprous iodide in 250 In a mixed solution of degassed toluene and 125 mL of degassed diisopropylamine, 105 mmol of trimethylsilylacetylene was added to the above reaction solution, and stirred for 24 h. After the reaction was complete, quenched by adding 250 mL of water, extracted with dichloromethane (3 x 100 mL), combined organic phase, Mg 2 SO 4 Dry, spin to dry the solvent, and purify by column chromatography (n-hexane) to give a pale yellow solid (yield: 42.5 mmol, 85%), 1 H NMR (400 MHz, CDCl 3 ): δ7.67(s, 2H), 0.27(s, 18H).
[0067] (2) Synthesis of raw material B, 1,4-bis(bis(trimethylphenylboron))-2,5-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com