Synthesis of phosphorescent iridium complex and application of phosphorescent iridium complex for fluorescence labeling of schistosome cercaria
A phosphorescent iridium complex, phosphorescent technology, applied in fluorescence/phosphorescence, indium organic compounds, platinum group organic compounds, etc., can solve the problems of technical difficulties, decrease in fluorescence intensity, photobleaching, etc., and achieve high luminescence quantum efficiency and long emission lifetime. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
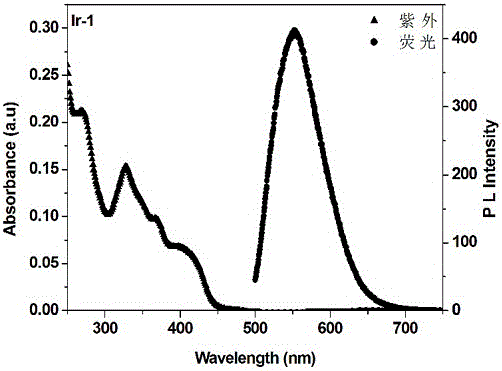
[0036] [Ir(PEG-bt) 2 (phen)][PF 6 ](Ir-1) Synthesis:
[0037]
[0038] (1) Synthesis of HO-bt: Add 1 mmol of 4-phenolboronic acid, 1 mmol of 2-chlorobenzothiazole, 7% equivalent tetrakis(3-phenylphosphine)palladium(0) and 1 mmol of potassium carbonate to tris Neck flask, then add 30mLTHF and H 2 O (V / V=1:1) mixed solvent. Under the protection of nitrogen, the reaction system was at 70 o C reacted for 24h. After cooling to room temperature, the reaction solution was extracted with dichloromethane, and the aqueous phase was extracted 3 times with dichloromethane (10 mL×3). The organic layers were combined, dried with anhydrous sodium sulfate, and the solvent was removed under reduced pressure, followed by column chromatography to obtain the product.
[0039] (2) Synthesis of triethylene glycol single-ended ester: Add 1 mmol of triethylene glycol into a three-neck flask, add 1 mmol of pyridine, and dissolve with 20 ml of dichloromethane. at 0 o C and stir in an ice bath...
Embodiment 2
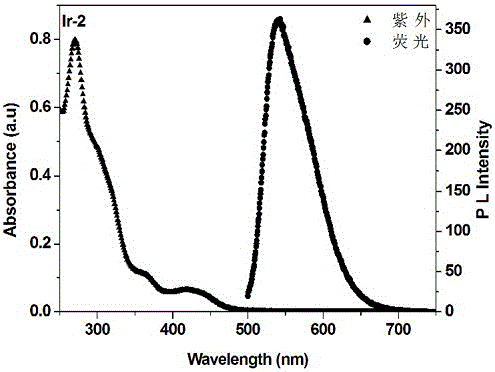
[0045] [Ir(CHO-ppy) 2 (phen)][PF 6 ](Ir-2) Synthesis:
[0046]
[0047] (1) Synthesis of ligand CHO-ppy: Add 1 mmol of 4-formylphenylboronic acid, 1 mmol of 2-chloropyridine, 7% equivalent tetrakis(3-phenylphosphine) palladium (0) and 1 mmol of potassium carbonate into a three-necked flask, and then add 50 mL of THF and H 2 O (V / V=1:1) mixed solvent. Under the protection of nitrogen, the reaction system was at 70 o C reacted for 24h. Cool to room temperature, extract the reaction solution with dichloromethane, extract the aqueous phase with dichloromethane three times (10mL×3), combine the organic layers, dry with anhydrous sodium sulfate overnight, remove the solvent under reduced pressure, and separate by column chromatography to obtain CHO -ppy.
[0048] (2) Synthesis of CHO-ppy iridium dichloro bridge complex (see literature Nonoyama, K. Bull. Chem. Soc. Jpn. 1974, 47, 467-468.).
[0049] (3) Add 0.25mmol of CHO-ppy iridium dichloro bridge complex and 0.5mmol of ...
Embodiment 3
[0051] [Ir(CHO-bt) 2 (phen)][PF 6 ](Ir-3) Synthesis:
[0052]
[0053] (1) Synthesis of ligand CHO-bt: 2 mmol of 4-formylphenylboronic acid, 2 mmol of 2-chlorobenzothiazole, 7% equivalent of tetrakis (3-phenylphosphine) palladium (0) and 2 mmol of carbonic acid Potassium was added to the three-necked flask, and then 45 mL of THF and H 2 O (V / V=1:1) mixed solvent. Under the protection of nitrogen, the reaction system was at 70 o C reacted for 24h. Cool to room temperature, extract the reaction solution with dichloromethane, extract the aqueous phase with dichloromethane 3 times (10mL×3), combine the organic layers, dry with anhydrous sodium sulfate overnight, remove the solvent under reduced pressure, and separate the product by column chromatography .
[0054] (2) Synthesis of CHO-bt iridium dichloro bridge complex (see literature Nonoyama, K. Bull. Chem. Soc. Jpn. 1974, 47, 467-468.).
[0055] (3) Add 0.9mmol of CHO-bt iridium dichloro bridge complex and 1.8mmol of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com