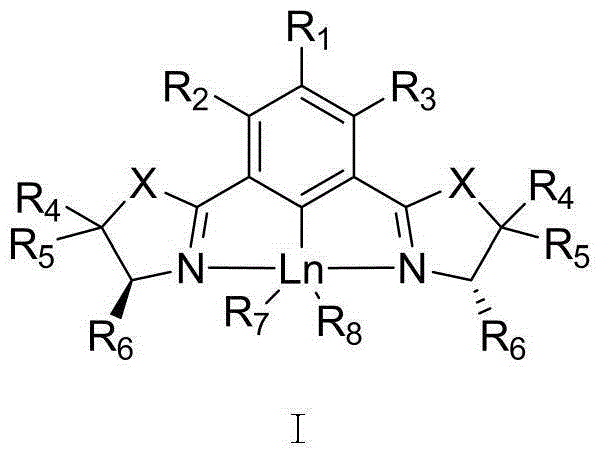
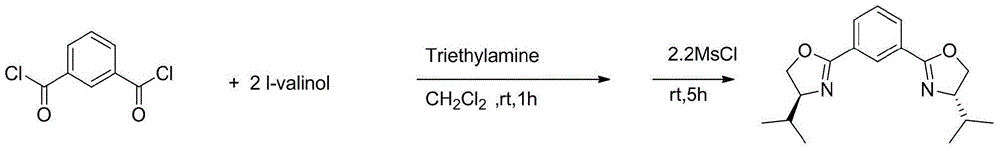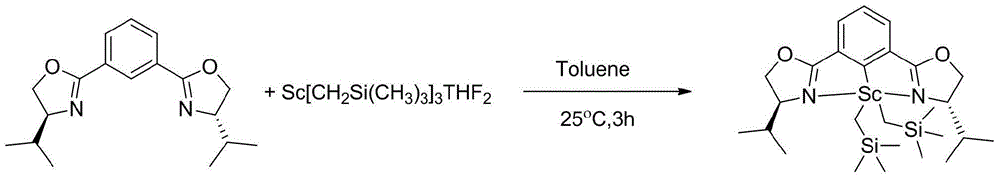Chiral NCN bisoxazoline phenyl rare earth metal catalyst, preparation method therefor and application thereof
A bisoxazoline phenyl rare earth and metal catalyst technology, applied in the field of catalysis, can solve the problems of rare polymers with high selectivity, and achieve the effects of high catalytic activity and selectivity, high economic efficiency and easy modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Preparation of chiral NCN bisoxazoline ligand [(S,S)-Phebox-iPr]H
[0047]
[0048] First, under a nitrogen atmosphere, isophthaloyl dichloride (1.02 g, 5 mmol) was added into a three-necked flask, dichloromethane was added to dissolve, and triethylamine (7.6 g, 75 mmol) was added. At 0 °C, a solution of L-valinol (1.03 g, 10 mmol) in dichloromethane was added. After the dropwise addition, return to room temperature and react for 1 h. Then methanesulfonyl chloride (1.26g, 11mmol) was slowly added dropwise at 0°C, and returned to room temperature to react for 5h after dropping. At 0°C, potassium carbonate aqueous solution was added to the reaction liquid to quench the reaction, the mixture was extracted with ethyl acetate, the organic layer was washed with saturated brine, and dried over sodium sulfate. Filter and spin off the solvent. The crude product was obtained by column chromatography 1.2g, and the yield was 74%.
[0049] (2)[(S,S)-Phebox-iPr]Sc(CH 2 Si...
Embodiment 2
[0053] (1) Preparation of chiral NCN bisoxazoline ligand [(S,S)-Phebox-iBu]H
[0054]
[0055] The specific steps are similar to the ligand [(S, S)-Phebox-iPr] H method in Example 1, except that L-valinol is replaced by L-leucinol to obtain ligand 1.15g, and the yield is 70%.
[0056] (2)[(S,S)-Phebox-iBu]Sc(CH 2 SiMe 3 ) 2 Catalyst preparation
[0057]
[0058] Concrete steps and [(S, S)-Phebox-iPr]Sc(CH 2 SiMe 3 ) 2 The method is similar, except that the ligand [(S,S)-Phebox-iPr]H is replaced by [(S,S)-Phebox-iBu]H. Get [(S,S)-Phebox-iBu]Sc(CH 2 SiMe 3 ) 2 Catalyst 1g, the yield is 60.24%.
Embodiment 3
[0060] (1) Preparation of chiral NCN bisoxazoline ligand [(S,S)-Phebox-Ph]H
[0061]
[0062] The specific steps are similar to the ligand [(S, S)-Phebox-iPr] H method in Example 1, except that L-valinol is replaced by L-phenadinol to obtain 1.1 g of the product, and the yield is 60%.
[0063] (2)[(S,S)-Phebox-Ph]Sc(CH 2 SiMe 3 ) 2 Catalyst preparation
[0064]
[0065] Concrete steps and [(S, S)-Phebox-iPr]Sc(CH 2 SiMe 3 ) 2 The method is similar, except that the ligand [(S,S)-Phebox-iPr]H is replaced by [(S,S)-Phebox-Ph]H to obtain [(S,S)-Phebox-Ph]Sc( CH 2 SiMe 3 ) 2 Catalyst 1.03g, yield 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com